P0380
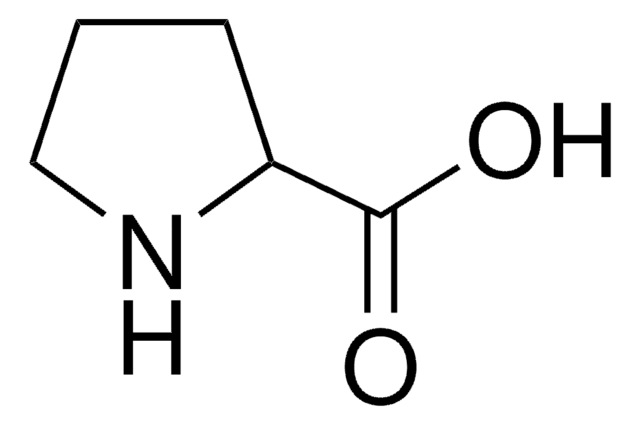
L-Proline
≥99% (HPLC), ReagentPlus®
Synonym(s):
(S)-Pyrrolidine-2-carboxylic acid
About This Item
Recommended Products
product name
L-Proline, ReagentPlus®, ≥99% (HPLC)
Quality Level
product line
ReagentPlus®
Assay
≥99% (HPLC)
form
powder
impurities
L-Hydroxyproline, free
color
white
mp
228 °C (dec.) (lit.)
application(s)
detection
SMILES string
OC(=O)[C@@H]1CCCN1
InChI
1S/C5H9NO2/c7-5(8)4-2-1-3-6-4/h4,6H,1-3H2,(H,7,8)/t4-/m0/s1
InChI key
ONIBWKKTOPOVIA-BYPYZUCNSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- As a supplement during the preparation of chondrogenic medium and synthetic dextrose minimal medium (SD).
- As a standard during the identification of metabolites in serum samples.
- In a study to prepare L-proline-L-phenylalanine (L-Pro-L-Phe) mixture in aqueous acetonitrile.
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Amino Acid Codon Wheel for fast RNA translation. Find which amino acid is translated from your RNA sequence quickly and easily.
Related Content
The List group focuses on the development of new concepts in catalysis. Since 1999, this research group has pioneered the development of organocatalysis as the third pillar of stereoselective catalysis, along side biocatalysis and transition metal catalysis.
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service