52596
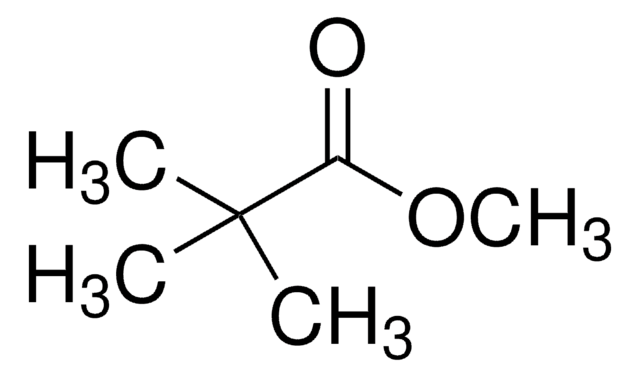
Methyl pivalate
suitable for GC/MS, ≥99.9% (GC)
Synonym(s):
Methyl 2,2-dimethylpropionate, Methyl trimethylacetate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)3CCOOCH3
CAS Number:
Molecular Weight:
116.16
Beilstein:
1744141
EC Number:
MDL number:
UNSPSC Code:
12190000
PubChem Substance ID:
NACRES:
NB.21
Recommended Products
Quality Level
Assay
≥99.9% (GC)
form
liquid
technique(s)
GC/MS: suitable
refractive index
n20/D 1.390 (lit.)
bp
101 °C (lit.)
mp
-70 °C
density
0.873 g/mL at 25 °C (lit.)
application(s)
food and beverages
SMILES string
COC(=O)C(C)(C)C
InChI
1S/C6H12O2/c1-6(2,3)5(7)8-4/h1-4H3
InChI key
CNMFHDIDIMZHKY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Methyl pivalate is a methyl ester derived from pivalic acid and methanol. It finds use as a solvent and an agent for transesterification reactions. It has gained attention for its positive environmental attributes. It exhibits a significantly lower ozone-forming potential compared to widely used oxygenated solvents like n-butyl ethanoate and butanol, as measured by a maximum incremental reactivity scale.
Application
Methyl pivalate can be used as a solvent for preparing the ISTD solution, diluting allergen standards, and aiding in the extraction of the sample components during the GC-MS analysis process.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
42.8 °F - closed cup
Flash Point(C)
6 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Quantum chemical investigation of the Koch carbonylation of methyl tert-butyl ether (MTBE)
Haubein NC, et al.
Industrial & Engineering Chemistry Research, 43(1), 18-29 (2004)
Online clean-up of volatile compounds in complex matrices for GC-MS quantification: testing with fragranced consumer products
Debonneville C and Chaintreau A
Flavour and Fragrance Journal, 29(5), 267-276 (2014)
13 C NMR Study of the Acid-Catalyzed Carbonylation of Methyl tert-Butyl Ether (MTBE)}
Haubein NC, et al.
Catalysis Letters, 80, 139-145 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service