15450
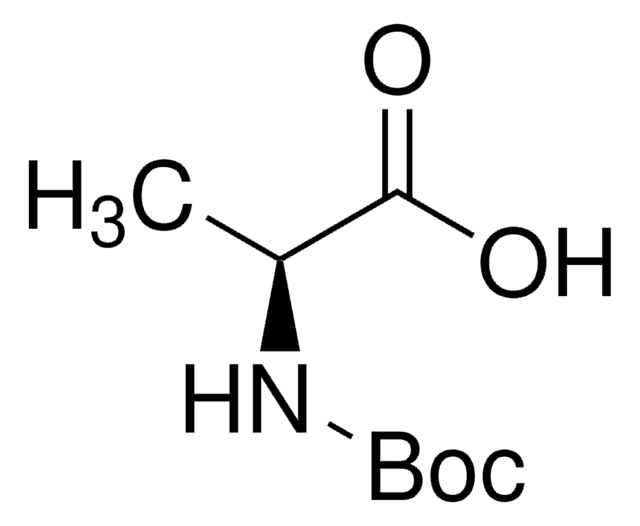
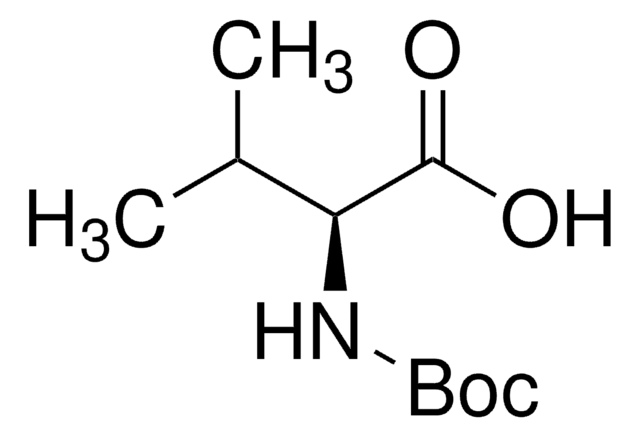
Boc-Leu-OH hydrate
≥99.0% (HPLC)
Synonym(s):
Boc-L-leucine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2CHCH2CH(COOH)NHCOOC(CH3)3 xH2O
Molecular Weight:
231.29 (anhydrous basis)
EC Number:
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99.0% (HPLC)
form
solid
optical activity
[α]20/D −25±0.5°, c = 2% in acetic acid
reaction suitability
reaction type: Boc solid-phase peptide synthesis
reaction type: C-H Activation
reagent type: ligand
reaction type: Peptide Synthesis
mp
85-90 °C
application(s)
peptide synthesis
functional group
amine
carboxylic acid
SMILES string
CC(C)C[C@H](NC(OC(C)(C)C)=O)C(O)=O
Application
Boc-Leu-OH (Boc-L-leucine) was used in the synthesis of a potent cytotoxin, PM-94128.
Boc-protected leucine (Boc-Leu-OH) can be used to generate combinatorial peptide libraries and also to synthesize peptide models to study structure-activity relationships.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Multicyclic polypeptide model compounds. 1. Synthesis of a tricyclic amphiphilic. alpha.-helical peptide using an oxime resin, segment-condensation approach.
Osapay G, et al.
Journal of the American Chemical Society, 112(16), 6046-6051 (1990)
Screening of mixture combinatorial libraries for chiral selectors: a reciprocal chromatographic approach using enantiomeric libraries.
Wu Y, et al.
Analytical Chemistry, 71(9), 1688-1691 (1999)
Masaru Enomoto et al.
The Journal of organic chemistry, 74(19), 7566-7569 (2009-09-03)
The enantioselective total synthesis of PM-94128, a potent cytotoxin of microbial origin, was accomplished by a concise nine-step sequence of reactions in 14% overall yield from N-Boc-l-leucine. The synthesis of Y-05460M-A, a one-carbon lower homologue of PM-94128, was also achieved
The synthesis and screening of a combinatorial peptide library for affinity ligands for glycosylated haemoglobin1.
Chen B, et al.
Biosensors And Bioelectronics, 13(7-8), 779-785 (1998)
Hyun-Woong Cho et al.
PloS one, 14(6), e0217745-e0217745 (2019-06-21)
The aim of this study was to investigate the short-term efficacy and safety of Poly-gamma-glutamic acid (γ-PGA) and the immunologic changes in patients with CIN 1. Participants were randomly assigned to one of two groups and orally treated with placebo
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service