495609
Okadaic Acid, Prorocentrum sp.
InSolution, ≥95%, inhibitor of protein phosphatase 1
Synonym(s):
InSolution Okadaic Acid, Prorocentrum sp.
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
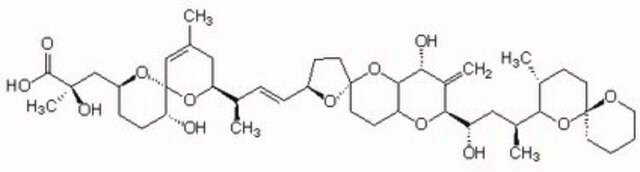
C44H68O13
Molecular Weight:
805.00
UNSPSC Code:
12352106
Recommended Products
Quality Level
Assay
≥95% (HPLC)
form
liquid
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
desiccated (hygroscopic)
protect from light
shipped in
wet ice
storage temp.
−20°C
General description
An ionophore-like polyether derivative of a C38 fatty acid compound isolated from dinoflagellates that have fed on the marine sponge Halinchrondria okadai. It is a potent non-comepetitive, reversible inhibitor of serine/threonine-specific protein phosphatases 1 (PP1, IC50 = 10-15 nM, rabbit skeletal muscle, catalytic subunit) and 2A (PP2A, IC50 = 0.1 nM, rabbit skeletal muscle, catalytic subunit). It has only a trivial effect on protein phosphatase 2B (IC50 = 5 µM), a Ca2+/calmodulin-dependent enzyme, while PP2C, a Mg2+ dependent enzyme, is unaffected. Okadaic acid has no significant effect on the activities of tyrosine phosphatases, alkaline phosphatase, acid phosphatase, and inositol trisphosphatase. It is also a non-phorbol ester-type tumor promoter on mouse skin. Useful for the study of protein phosphatases and of PP1/PP2A in cell extracts, as well as in intact cells. Induces apoptosis in human breast carcinoma (MB-231 and MCF-7) and in myeloid cells, but inhibits glucocorticoid-induced apoptosis in T-cell hybridomas. Has marked contractile effect on smooth muscles and heart muscles. Implicated as a causative agent of diarrhetic shellfish poisoning.
Biochem/physiol Actions
Cell permeable: no
Primary Target
protein phosphatase
protein phosphatase
Product does not compete with ATP.
Reversible: no
Target IC50: 10-15 nM against protein phosphatase 1; 0.1 nM against protein phosphatase 2A
Packaging
Packaged under inert gas
Warning
Toxicity: Toxic (F)
Physical form
A 250 µM (25 µg/124 µl) solution of Okadaic Acid, (Cat. No. 495604) in DMSO.
Reconstitution
Following initial thaw, aliquot and freeze (-20°C).
Other Notes
Due to the nature of the Hazardous Materials in this shipment, additional shipping charges may be applied to your order. Certain sizes may be exempt from the additional hazardous materials shipping charges. Please contact your local sales office for more information regarding these charges.
Gjertsen, B.T., et al. 1994. J. Cell Sci.107, 3363.
Kiguchi, K., et al. 1994. Cell Growth Differentiation5, 995.
Ohaka, Y., et al. 1993. Biochem. Biophys. Res. Commun. 197, 916.
Gopalakrishna, R., et al. 1992. Biochem. Biophys. Res. Commun. 189, 950.
Kreienbuhl, P., et al. 1992. Blood80, 2911.
Nomura, M., et al. 1992. Biochemistry31, 11915.
Song, Q., et al. 1992. J. Cell Physiol.153, 550.
Tada, Y., et al. 1992. Immunopharmacol.24, 17.
Cohen, P., et al. 1990. Trends Biochem. Sci.15, 98.
Cohen, P. 1989. Annu. Rev. Biochem.58, 453.
Cohen, P., and Cohen, P.T. 1989. J. Biol. Chem.264, 21435.
Haystead, T.A., et al. 1989. Nature337, 78.
Kiguchi, K., et al. 1994. Cell Growth Differentiation5, 995.
Ohaka, Y., et al. 1993. Biochem. Biophys. Res. Commun. 197, 916.
Gopalakrishna, R., et al. 1992. Biochem. Biophys. Res. Commun. 189, 950.
Kreienbuhl, P., et al. 1992. Blood80, 2911.
Nomura, M., et al. 1992. Biochemistry31, 11915.
Song, Q., et al. 1992. J. Cell Physiol.153, 550.
Tada, Y., et al. 1992. Immunopharmacol.24, 17.
Cohen, P., et al. 1990. Trends Biochem. Sci.15, 98.
Cohen, P. 1989. Annu. Rev. Biochem.58, 453.
Cohen, P., and Cohen, P.T. 1989. J. Biol. Chem.264, 21435.
Haystead, T.A., et al. 1989. Nature337, 78.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
188.6 °F - closed cup - (Dimethylsulfoxide)
Flash Point(C)
87 °C - closed cup - (Dimethylsulfoxide)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Ilse Delint-Ramirez et al.
Molecular cell, 82(20), 3794-3809 (2022-10-08)
Neuronal activity induces topoisomerase IIβ (Top2B) to generate DNA double-strand breaks (DSBs) within the promoters of neuronal early response genes (ERGs) and facilitate their transcription, and yet, the mechanisms that control Top2B-mediated DSB formation are unknown. Here, we report that
Julia Kamenz et al.
Current biology : CB, 31(4), 794-808 (2020-12-29)
The phosphorylation of mitotic proteins is bistable, which contributes to the decisiveness of the transitions into and out of M phase. The bistability in substrate phosphorylation has been attributed to bistability in the activation of the cyclin-dependent kinase Cdk1. However
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service