05-298
Anti-α-Dystroglycan Antibody, clone VIA4-1
culture supernatant, clone VIA4-1, Upstate®
Synonym(s):
Anti-156DAG, Anti-A3a, Anti-AGRNR, Anti-DAG, Anti-LGMDR16, Anti-MDDGA9, Anti-MDDGC7, Anti-MDDGC9
About This Item
Recommended Products
biological source
mouse
Quality Level
antibody form
culture supernatant
antibody product type
primary antibodies
clone
VIA4-1, monoclonal
species reactivity
guinea pig, rabbit, rat, human, mouse, canine
manufacturer/tradename
Upstate®
technique(s)
immunohistochemistry: suitable
western blot: suitable
isotype
IgG1
NCBI accession no.
UniProt accession no.
shipped in
wet ice
target post-translational modification
unmodified
Gene Information
human ... DAG1(1605)
General description
Dystroglycan is widely distributed in non-muscle tissues as well as in muscle tissues. During epithelial morphogenesis of kidney, the dystroglycan complex is shown to act as a receptor for the basement membrane. Dystroglycan expression in Mus musculus brain and neural retina has also been reported. However, the physiological role of dystroglycan in non-muscle tissues has remained unclear.
Specificity
Immunogen
Application
Cell Structure
Cytoskeleton
Quality
Target description
Physical form
Storage and Stability
Analysis Note
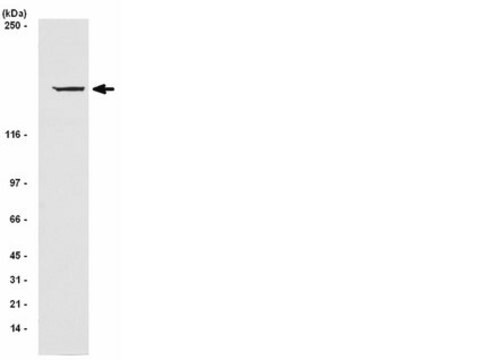
Mouse, rat and human skeletal muscle tissue extracts or DU 145 (human prostate carcinoma tumor) cell lysate
Other Notes
Legal Information
Disclaimer
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 2
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service