671576
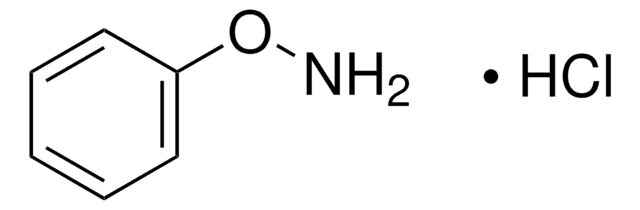
N-Phenylhydroxylamine
≥95.0%
Synonym(s):
N-Hydroxyaniline, N-Hydroxybenzenamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H7NO
CAS Number:
Molecular Weight:
109.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥95.0%
form
solid
mp
80-84 °C
storage temp.
−20°C
SMILES string
ONc1ccccc1
InChI
1S/C6H7NO/c8-7-6-4-2-1-3-5-6/h1-5,7-8H
InChI key
CKRZKMFTZCFYGB-UHFFFAOYSA-N
Related Categories
Application
N-Phenylhydroxylamine can be used as a starting material for the synthesis of:
- 2-alkylindoles by treating with aliphatic terminal alkynes using gold catalyst via sequential 3,3-rearrangements and cyclodehydrations.
- Isoxazolidines by reacting with aldehydes and α, β-unsaturated aldehydes via a three-component one-pot catalytic reaction.
- Tetrahydro-1,2-oxazines by treating with an aldehyde and cyclopropane via homo 3+2 dipolar cycloaddition reaction.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Copper-catalyzed amination of alkenes and ketones by phenylhydroxylamine.
Ho C-M and Lau T-C
New. J. Chem., 24(11), 859-863 (2000)
Nilanjana Chowdhury et al.
Bioorganic & medicinal chemistry letters, 20(18), 5414-5417 (2010-08-21)
Photoinduced homolytic fission of nitrogen-oxygen bond in N,O-diacyl-4-benzoyl-N-phenylhydroxylamines using 310 nm UV light for 10 min produced acylaminyl and acyloxy radicals, which resulted in single strand cleavage of DNA at pH 7.0. Further the DNA cleaving ability of N,O-diacyl-4-benzoyl-N-phenylhydroxylamines found
T P Bradshaw et al.
Free radical biology & medicine, 18(2), 279-285 (1995-02-01)
Previous studies have shown that incubation of rat red blood cells in vitro with phenylhydroxylamine (50-300 microM) induces rapid splenic sequestration of the red cells on reintroduction to isologous rats. EPR and the spin trapping agent, 5,5-dimethyl-1-pyrroline-N-oxide (DMPO), were utilized
Y Endo et al.
Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan, 114(8), 565-576 (1994-08-01)
The acid-catalyzed reaction of N-acyl- and N-sulfonylhydroxylamines with benzene proceeded smoothly to give C-C products; 2- and 4-hydroxybiphenyls. The reaction and the thermolysis of N-aryloxypyridinium salts involve common intermediates. The results of product analysis, the orientation of the reaction, effects
M F Khan et al.
Toxicological sciences : an official journal of the Society of Toxicology, 42(1), 64-71 (1998-05-30)
To elucidate the role of N-phenylhydroxylamine (PHA, N-hydroxylated metabolite of aniline) in the selective toxicity of aniline to the spleen, dose-dependent studies were conducted with PHA in rats. Male Sprague-Dawley rats were given four doses each (1 dose/day) of 0.025
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service