All Photos(3)
About This Item
Empirical Formula (Hill Notation):
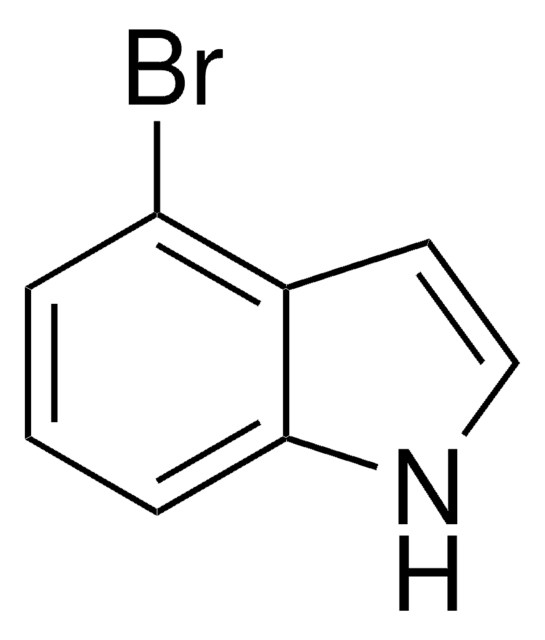
C8H6BrN
CAS Number:
Molecular Weight:
196.04
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
solid
mp
41-44 °C (lit.)
SMILES string
Brc1cccc2cc[nH]c12
InChI
1S/C8H6BrN/c9-7-3-1-2-6-4-5-10-8(6)7/h1-5,10H
InChI key
RDSVSEFWZUWZHW-UHFFFAOYSA-N
Related Categories
General description
7-Bromoindole is a 7-substituted indole derivative. Its synthesis from 7-bromoindole-2-carboxylic acid has been reported. It has been reported to reduce the production of staphyloxanthin in Staphylococcus aureus.
Application
7-Bromoindole may be used in the synthesis of the following:
- indole
- dyestuffs
- 8-bromocarboline
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Total synthesis of indoles from Tricholoma species via Bartoli/heteroaryl radical methodologies.
A Dobbs
The Journal of organic chemistry, 66(2), 638-641 (2001-06-30)
Zhiqian Wang et al.
Tetrahedron letters, 53(5), 477-479 (2012-05-01)
A novel MCAP-cycloaddition sequence has been applied to the facile synthesis of β-carboline intermediates to gain rapid access to novel derivatives of yohimbine-like and corynanthe-like compounds that may be easily diversified by cross-coupling reactions and N-derivatizations to generate small compound
The structure of monobrominated ethyl indole-3-carboxylate and the preparation of 7-bromoindole.
Leggetter BE and Brown RK.
Canadian Journal of Chemistry, 38(9), 1467-1471 (1960)
Jin-Hyung Lee et al.
Applied microbiology and biotechnology, 97(10), 4543-4552 (2013-01-16)
Human pathogens can readily develop drug resistance due to the long-term use of antibiotics that mostly inhibit bacterial growth. Unlike antibiotics, antivirulence compounds diminish bacterial virulence without affecting cell viability and thus, may not lead to drug resistance. Staphylococcus aureus
J Y Kim et al.
Letters in applied microbiology, 41(2), 163-168 (2005-07-22)
To establish multicomponent phenol hydroxylases (mPHs) as novel biocatalysts for producing dyestuffs and hydroxyindoles such as 7-hydroxyindole (7-HI) from indole and its derivatives. We have isolated Pseudomonas sp. KL33, which possesses a phenol degradation pathway similar to that found in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service