All Photos(2)
About This Item
Empirical Formula (Hill Notation):
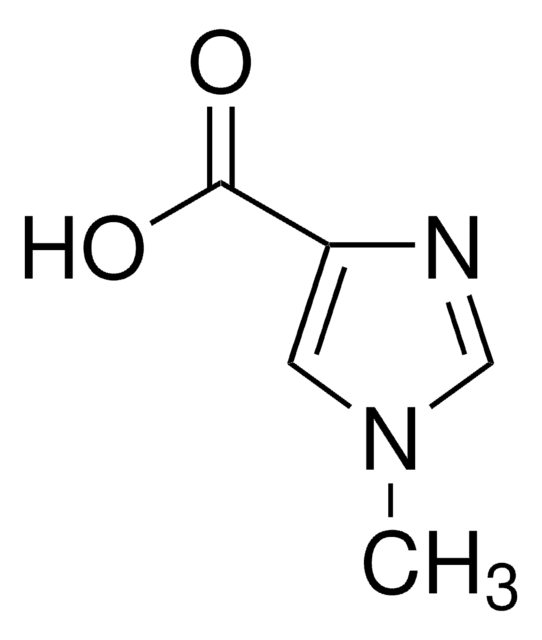
C4H4N2O2
CAS Number:
Molecular Weight:
112.09
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
294-295 °C (lit.)
SMILES string
OC(=O)c1c[nH]cn1
InChI
1S/C4H4N2O2/c7-4(8)3-1-5-2-6-3/h1-2H,(H,5,6)(H,7,8)
InChI key
NKWCGTOZTHZDHB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
4-Imidazolecarboxylic acid (1H-imidazole-4-carboxylic acid, H2imc) is an imidazole derivative that contains an imidazole group and a carboxylate group. It has been widely utilized to generate different types of coordination polymers. The anion of 4-imidazolecarboxylic acid has been reported to stabilize binuclear hydroxo complexes of trivalent lanthanides in the pH range 7-10.
Application
4-Imidazolecarboxylic acid (ICA) was used in the synthesis of triphenymethyl-protected 4-imidazole carboxylic acid (trityl-ImCOOH) which is employed to functionalize poly(propylene imine) dendrimers.
It may be used in the following studies:
It may be used in the following studies:
- In the synthesis of lanthanide sulfate–carboxylates, [Ln(HIMC)(SO4)(H2O)] (Ln = Dy and Eu, HIMC = 4-imidazolecarboxylic acid) by in situ decarboxylation in the presence of Cu(II) ions.
- As an internal standard to obtain calibration curve by plotting the peak area ratios of histamine (HA) and several metabolites isolated from C3H/HeNCrj mice hair relative to ICA.
- In the synthesis of tetranuclear manganese carboxylate complexes possessing imidazole-based N/O chelating ligands.
Other Notes
For a review of imidazoles, see Science of Synthesis.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Francisco Aguilar-Pérez et al.
Inorganic chemistry, 45(23), 9502-9517 (2006-11-07)
The anion of 4-imidazolecarboxylic acid (HL) stabilizes hydroxo complexes of trivalent lanthanides of the type ML(OH)+ (M = La, Pr) and M2L(n)(OH)(6-n) (M = La, n = 2; M = Pr, n = 2, 3; M = Nd, Eu, Dy
Synthesis and catalytic properties of imidazole-functionalized poly (propylene imine) dendrimers.
Baker LA, et al.
Bull. Korean Chem. Soc., 23(5), 647-654 (2002)
Tetranuclear manganese carboxylate clusters with imidazole-carboxylate chelating ligands. X-ray crystal structure of the 4-imidazoleacetate complex.
Boskovic C, et al.
Polyhedron, 19(18), 2111-2118 (2000)
catena-Poly [copper (I)-bis [μ-3-(1H-imidazol-2-yl) pyridine]-copper (I)-di-μ-iodido].
425842
Acta Crystallographica Section E, Structure Reports Online, 67(9), 1288-1288 (2011)
Diaquabis(1H-imidazole-4-carboxylato-κ2N3,O4) cobalt (II).
Chen WS.
Acta Crystallographica Section E, Structure Reports Online, 68(10), m1246-m1246 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service