415944
2-Amino-4′-methoxyacetophenone hydrochloride
90%, technical
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
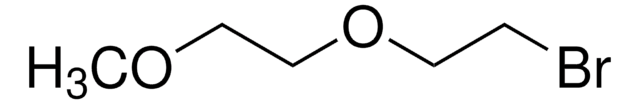
CH3OC6H4C(O)CH2NH2 · HCl
CAS Number:
Molecular Weight:
201.65
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical
Assay
90%
form
powder
mp
190-193 °C (lit.)
SMILES string
Cl.COc1ccc(cc1)C(=O)CN
InChI
1S/C9H11NO2.ClH/c1-12-8-4-2-7(3-5-8)9(11)6-10;/h2-5H,6,10H2,1H3;1H
InChI key
FZVYWBMMOSHMRS-UHFFFAOYSA-N
General description
2-Amino-4′-methoxyacetophenone hydrochloride is an organic building block.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Meredith C Henderson et al.
Molecular cancer therapeutics, 8(1), 36-44 (2009-01-14)
Pancreatic carcinoma is the fourth leading cause of death from cancer. Novel targets and therapeutic options are needed to aid in the treatment of pancreatic cancer. The compound UA62784 is a novel fluorenone with inhibitory activity against the centromere protein
Arthur Y Shaw et al.
The Journal of pharmacology and experimental therapeutics, 331(2), 636-647 (2009-08-07)
A series of diaryl- and fluorenone-based analogs of the lead compound UA-62784 [4-(5-(4-methoxyphenyl)oxazol-2-yl)-9H-fluoren-9-one] was synthesized with the intention of improving upon the selective cytotoxicity of UA-62784 against human pancreatic cancer cell lines with a deletion of the tumor suppressor gene
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service