All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C4H4BrN3
CAS Number:
Molecular Weight:
174.00
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
241-243 °C (lit.)
SMILES string
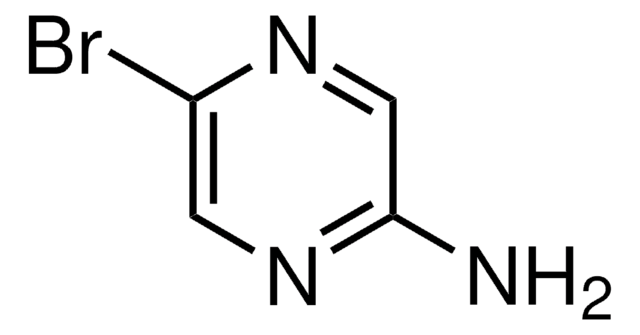
Nc1ncc(Br)cn1
InChI
1S/C4H4BrN3/c5-3-1-7-4(6)8-2-3/h1-2H,(H2,6,7,8)
InChI key
UHRHPPKWXSNZLR-UHFFFAOYSA-N
Related Categories
General description
Crystal structure of 2-amino-5-bromopyrimidine was studied.
Application
2-Amino-5-bromopyrimidine was employed:
- in synthesis of pyridine, pyrimidine and pyridinone C-nucleoside phosphoramidites
- in synthesis of 2-phthalimido-5-bromopyrimidine
- as intermediate for the preparation of sulfanilamides and amino acids containing the pyrimidine ring system. The products are potential antiviral agents
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Structure of 2-amino-5-bromopyrimidine.
Watton HLL, et al.
Acta Crystallographica Section C, Crystal Structure Communications, 44(10), 1857-1858 (1988)
Journal of Chemical Education, 62, 905-905 (1985)
Self-assembly of 1-and 2-dimensional multicompartmental arrays via the 2-aminopyrimidine H-bonding motif and selective guest inclusion.
Krische MJ, et al.
Tetrahedron, 56(36), 6701-6706 (2000)
Jun Lu et al.
The Journal of organic chemistry, 74(21), 8021-8030 (2009-10-02)
In the structures of the HDV ribozyme a cytosine nucleobase resides at the active site poised to participate directly in catalysis. Defining the functional role of the nucleobase requires nucleoside analogues that perturb the functional groups in a strategic manner.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service