228435
5-Bromopyridine-3-carboxylic acid
98%
Synonym(s):
5-Bromonicotinic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C6H4BrNO2
CAS Number:
Molecular Weight:
202.01
Beilstein:
115854
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
178-180 °C (lit.)
SMILES string
OC(=O)c1cncc(Br)c1
InChI
1S/C6H4BrNO2/c7-5-1-4(6(9)10)2-8-3-5/h1-3H,(H,9,10)
InChI key
FQIUCPGDKPXSLL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
5-Bromopyridine-3-carboxylic acid (5-bromonicotinic acid) was used in the synthesis of 3-guanidinomethyl-5-iodopyridine.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
G Vaidyanathan et al.
Bioconjugate chemistry, 9(6), 758-764 (1998-11-17)
Substituting a pyridine ring for a benzene ring in the acylation agent N-succinimidyl 3-iodobenzoate has resulted in a useful approach for the radiohalogenation of monoclonal antibodies, peptides, and labeled biotin conjugates. It was hypothesized that such a substitution in m-iodobenzylguanidine
Małgorzata Dukat et al.
European journal of pharmacology, 435(2-3), 171-180 (2002-02-01)
Two 5-substituted derivatives of nicotine (nicotinic acetylcholine receptor: K(i)=2.4 nM) were synthesized and evaluated: 5-bromonicotine (K(i)=6.9 nM) and 5-methoxynicotine (K(i)=14.3 nM). Despite their high affinity, neither 5-bromonicotine nor 5-methoxynicotine mimicked nicotine in producing antinociceptive (tail-flick, hotplate), hypolocomotor, or hypothermic effects
F Gabor et al.
Journal of pharmaceutical sciences, 84(9), 1120-1125 (1995-09-01)
Two types of monoclonal antibodies were used for the determination of nicergoline in biological matrices. The antibodies were prepared with the hydrolysis products 5-bromonicotinic acid and 1-methyl-10 alpha-methoxydihydrolysergol after hemisuccinoylation to haptens. The current amide bond-generating methods (mixed anhydride-, carbodiimide-
Tawfik Gharbaoui et al.
Bioorganic & medicinal chemistry letters, 17(17), 4914-4919 (2007-06-26)
A strategy for lead identification of new agonists of GPR109a, starting from known compounds shown to activate the receptor, is described. Early compound triage led to the formulation of a binding hypothesis and eventually to our focus on a series
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service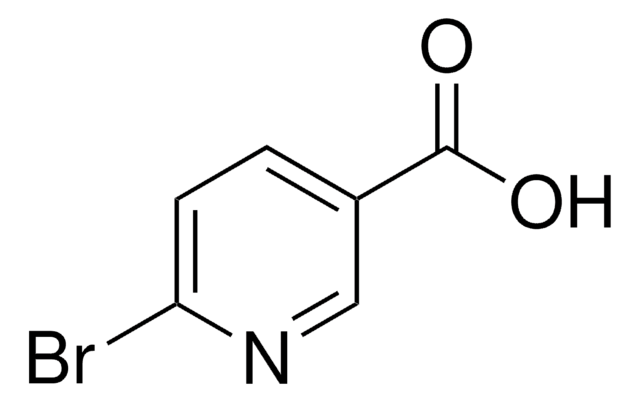





![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

