V900610
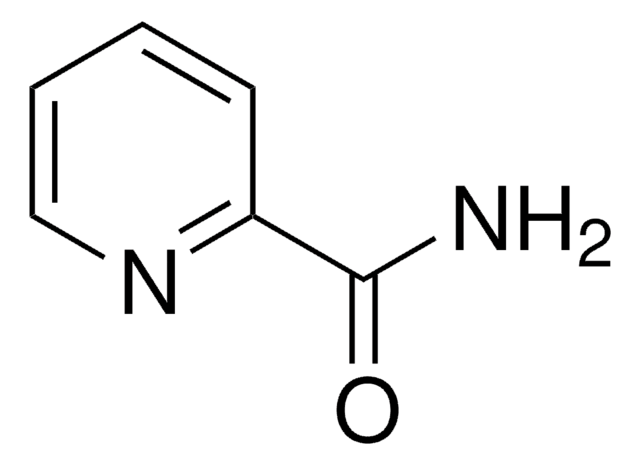
Isonicotinamide
Vetec™, reagent grade, 98%
Synonym(s):
Pyridine-4-carboxylic acid amide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C6H6N2O
CAS Number:
Molecular Weight:
122.12
Beilstein:
2173
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
grade
reagent grade
product line
Vetec™
Assay
98%
mp
155-157 °C (lit.)
SMILES string
NC(=O)c1ccncc1
InChI
1S/C6H6N2O/c7-6(9)5-1-3-8-4-2-5/h1-4H,(H2,7,9)
InChI key
VFQXVTODMYMSMJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Legal Information
Vetec is a trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Srinu Tothadi et al.
Philosophical transactions. Series A, Mathematical, physical, and engineering sciences, 370(1969), 2900-2915 (2012-05-23)
The idea of a structural landscape is based on the fact that a large number of crystal structures can be associated with a particular organic molecule. Taken together, all these structures constitute the landscape. The landscape includes polymorphs, pseudopolymorphs and
Yan Li et al.
European journal of pharmacology, 609(1-3), 13-18 (2009-03-17)
The phytoalexin resveratrol has been described to have chemopreventive and chemotherapeutic effects in several tumor models while its effects on osteosarcoma have not been extensively studied. Additionally, resveratrol is a potent activator of the Sirt1/Sir2 (silent information regulator 2) family
Jinjing Li et al.
Chemical communications (Cambridge, England), 47(5), 1530-1532 (2010-11-23)
For each of the well-known co-crystal formers, isonicotinamide and nicotinamide, a new polymorph, obtained during attempted co-crystallisation experiments, has been fully characterized and its stability relationship with previously reported forms established.
Jianqi Yang et al.
The Journal of biological chemistry, 284(40), 27042-27053 (2009-08-05)
The SIRT1 activators isonicotinamide (IsoNAM), resveratrol, fisetin, and butein repressed transcription of the gene for the cytosolic form of phosphoenolpyruvate carboxykinase (GTP) (PEPCK-C). An evolutionarily conserved binding site for hepatic nuclear factor (HNF) 4alpha (-272/-252) was identified, which was required
Joseph A Baur
Biochimica et biophysica acta, 1804(8), 1626-1634 (2009-11-10)
SIRT1 is the closest mammalian homologue of enzymes that extend life in lower organisms. Its role in mammals is incompletely understood, but includes modulation of at least 34 distinct targets through its nicotinamide adenine dinucleotide (NAD(+))-dependent deacetylase activity. Recent experiments
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service