S8139
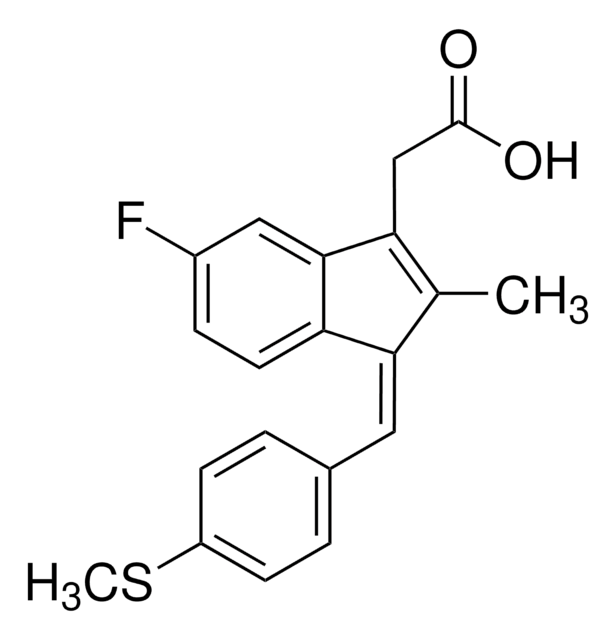
Sulindac
≥98.0%
Synonym(s):
(Z)-5-Fluoro-2-methyl-1-[p-(methylsulfinyl)benzylidene]indene-3-acetic acid
About This Item
Recommended Products
biological source
synthetic (organic)
Assay
≥98.0%
form
powder
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
solubility
methanol: 50 mg/mL
application(s)
forensics and toxicology
veterinary
originator
Merck & Co., Inc., Kenilworth, NJ, U.S.
SMILES string
CC1=C(CC(O)=O)c2cc(F)ccc2\C1=C/c3ccc(cc3)S(C)=O
InChI
1S/C20H17FO3S/c1-12-17(9-13-3-6-15(7-4-13)25(2)24)16-8-5-14(21)10-19(16)18(12)11-20(22)23/h3-10H,11H2,1-2H3,(H,22,23)/b17-9-
InChI key
MLKXDPUZXIRXEP-MFOYZWKCSA-N
Gene Information
human ... ALB(213) , PTGS1(5742) , PTGS2(5743)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Biochem/physiol Actions
Features and Benefits
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Repr. 2 - Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Protein-based drug transporters are expressed in Sf9 cells. Understanding the specific mechanisms of tumor cell transporters is an essential aspect of chemotherapeutic drug design.
Discover Bioactive Small Molecules for ADME/Tox
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service