PZ0004
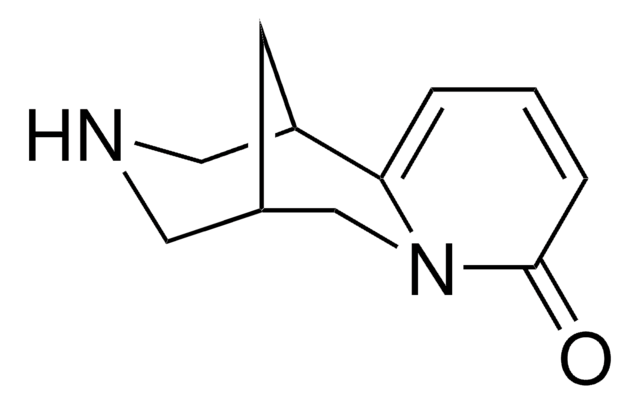
Varenicline tartrate
≥98% (HPLC)
Synonym(s):
7,8,9,10-Tetrahydro-6,10-methano-6H-pyrazino[2,3-h][3]benzazepine tartrate, Champix
About This Item
Recommended Products
Assay
≥98% (HPLC)
form
powder
color
white to beige
solubility
DMSO: >5 mg/mL
storage temp.
room temp
SMILES string
O[C@H]([C@@H](O)C(O)=O)C(O)=O.C1NC[C@H]2C[C@@H]1c3cc4nccnc4cc23
InChI
1S/C13H13N3.C4H6O6/c1-2-16-13-5-11-9-3-8(6-14-7-9)10(11)4-12(13)15-1;5-1(3(7)8)2(6)4(9)10/h1-2,4-5,8-9,14H,3,6-7H2;1-2,5-6H,(H,7,8)(H,9,10)/t8-,9+;1-,2-/m.1/s1
InChI key
TWYFGYXQSYOKLK-CYUSMAIQSA-N
Application
Biochem/physiol Actions
Features and Benefits
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Sigma-Aldrich offers many products related to nicotinic acetylcholine receptors for your research needs.
Discover Bioactive Small Molecules for ADME/Tox
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service