BCR140
Benzo[c]chrysene
BCR®, certified reference material
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C22H14
CAS Number:
Molecular Weight:
278.35
Beilstein:
1876196
MDL number:
UNSPSC Code:
41116107
PubChem Substance ID:
NACRES:
NA.24
Recommended Products
grade
certified reference material
Agency
BCR®
manufacturer/tradename
JRC
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
format
neat
storage temp.
2-8°C
SMILES string
c1ccc2c(c1)ccc3c2ccc4ccc5ccccc5c34
InChI
1S/C22H14/c1-3-7-18-15(5-1)11-14-21-20(18)13-12-17-10-9-16-6-2-4-8-19(16)22(17)21/h1-14H
InChI key
YZWGEMSQAMDWEM-UHFFFAOYSA-N
Analysis Note
For more information please see:
BCR140
BCR140
Legal Information
BCR is a registered trademark of European Commission
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A Pal et al.
FEBS letters, 486(2), 163-166 (2000-12-13)
Carcinogenic activity of many polycyclic aromatic hydrocarbons (PAHs) is mainly attributed to their respective diol epoxides, which can be classified as either bay or fjord region depending upon the location of the epoxide function. The Pi class human glutathione (GSH)
Federica Nalin et al.
Analytical and bioanalytical chemistry, 410(3), 1123-1137 (2017-11-10)
Retention indices (I) for 45 polycyclic aromatic hydrocarbons (PAHs) and 63 methyl-substituted PAHs were determined by gas chromatography - mass spectrometry (GC-MS) using two different stationary phases: a Rxi-PAH phase (a "higher phenyl-content stationary phase") and a 50% (mole fraction)
A S Giles et al.
Chemical research in toxicology, 10(11), 1275-1284 (1997-12-24)
The metabolic activation in mouse skin of benzo[c]chrysene (B[c]C), a weakly carcinogenic polycyclic aromatic hydrocarbon (PAH) present in coal tar and crude oil, was investigated. Male Parkes mice were treated topically with 0.5 mumol of B[c]C, and DNA was isolated
A Luch et al.
Carcinogenesis, 19(4), 639-648 (1998-05-26)
Metabolic activation of the racemic benzo[c]chrysene-trans-9,10-, benzo[g]chrysene-trans-11,12- and dibenzo[a,l]pyrene-trans-11,12-dihydrodiols to fjord region syn- and anti-dihydrodiol epoxides by microsomes of Aroclor 1254-treated Sprague-Dawley rats has been examined. Since the fjord region dihydrodiol epoxides were hydrolytically unstable under the experimental conditions, their
Shantu Amin et al.
Chemical research in toxicology, 16(2), 227-231 (2003-02-18)
Benzo[c]chrysene (BcC), an environmental pollutant, is a unique polycyclic aromatic hydrocarbon that possesses both a bay region and a fjord region in the same molecule. We previously demonstrated that both bay region and fjord region terminal rings are involved in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service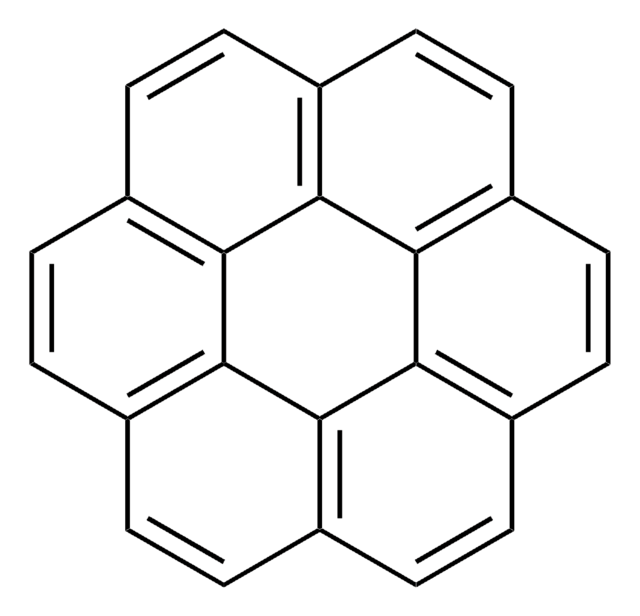

![Benzo[a]pyrene solution 100 μg/mL in cyclohexane, analytical standard](/deepweb/assets/sigmaaldrich/product/structures/253/820/be96d879-1811-46c0-8f11-612019691c2d/640/be96d879-1811-46c0-8f11-612019691c2d.png)