For information on the use of phenacyl bromide to derivatize carboxylic acids (e.g., fatty acids) for HPLC analysis, please see one or more of the following three references:1. K.J. Longmuir, et al., Determination of monoenoic fatty acid double bond position by permanganate-periodate oxidation followed by high-performance liquid chromatography of carboxylic acid phenacyl esters. Analytical Biochemistry, 167(2), 213-221 (1987).2. T. Hanis et al., Determination of fatty acids as phenacyl esters in rat adipose tissue and blood vessel walls by high-performance liquid chromatography. Journal of Chromatography, 452, 443-457 (1988). 3. A. Mehta et al., Rapid quantitation of free fatty acids in human plasma by high-performance liquid chromatography. Journal of Chromatography B Biomedical Science Applications, 719(1-2), 9-23 (1998).
77450
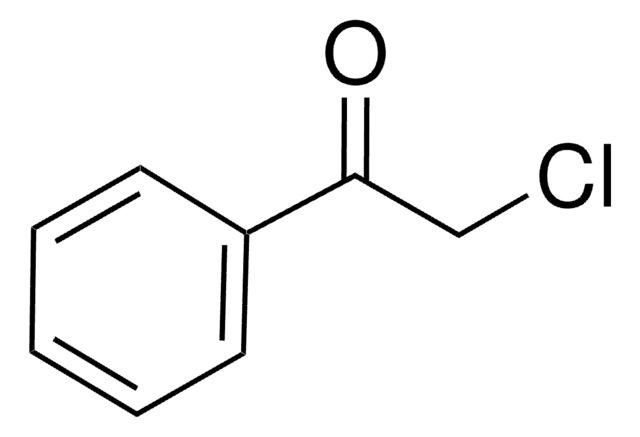
2-Bromoacetophenone
for GC derivatization, LiChropur™, ≥99.0%
Synonym(s):
ω-Bromoacetophenone, Phenacyl bromide
Select a Size
About This Item
Recommended Products
grade
for GC derivatization
Quality Level
Assay
≥99.0% (GC)
≥99.0%
form
crystals
quality
LiChropur™
reaction suitability
reagent type: derivatization reagent
reaction type: Acylations
technique(s)
gas chromatography (GC): suitable
bp
135 °C/18 mmHg (lit.)
mp
48-51 °C (lit.)
49-50 °C
storage temp.
2-8°C
SMILES string
BrCC(=O)c1ccccc1
InChI
1S/C8H7BrO/c9-6-8(10)7-4-2-1-3-5-7/h1-5H,6H2
InChI key
LIGACIXOYTUXAW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
-
Do you have references on the use of 2-Bromoacetophenone (also known as phenacyl bromide) Products 115835 and 77450 for making phenacyl esters of carboxylic acids for HPLC analysis?
1 answer-
Helpful?
-
-
What is the Department of Transportation shipping information for this product?
1 answer-
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Helpful?
-
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service