08581
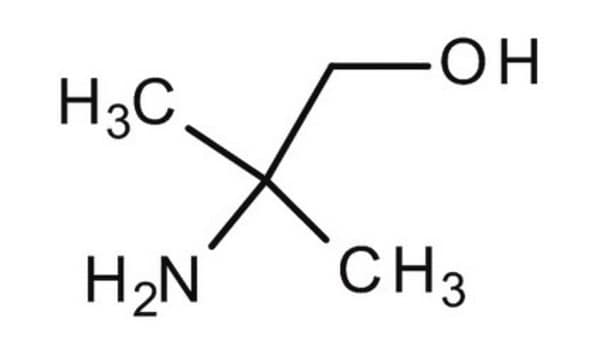
2-Amino-2-methyl-1-propanol
technical, ≥90% (GC)
Synonym(s):
β-Aminoisobutyl alcohol, AMP
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2C(NH2)CH2OH
CAS Number:
Molecular Weight:
89.14
Beilstein:
505979
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
3 (vs air)
Quality Level
vapor pressure
<1 mmHg ( 25 °C)
grade
technical
Assay
≥90% (GC)
form
solid
refractive index
n20/D 1.4455 (lit.)
useful pH range
9.0-10.5
pKa (25 °C)
9.7
bp
165 °C (lit.)
mp
24-28 °C (lit.)
density
0.934 g/mL at 25 °C (lit.)
SMILES string
CC(C)(N)CO
InChI
1S/C4H11NO/c1-4(2,5)3-6/h6H,3,5H2,1-2H3
InChI key
CBTVGIZVANVGBH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
2-Amino-2-methyl-1-propanol (AMP) is a sterically hindered amine that can be used:
- As an acid gas treating solvent to remove CO2 and H2S from gas streams by absorption.
- As a reagent in the synthesis of a fluorophore, silicon-rhodamine dye, for bioimaging.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Eye Dam. 1 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
179.8 °F - closed cup
Flash Point(C)
82.1 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A near-infrared fluorophore for live-cell super-resolution microscopy of cellular proteins.
Lukinavicius, G, et al.
Nature Chemistry, 5(2), 132-132 (2013)
Characterization and comparison of the CO2 absorption performance into single and blended alkanolamines in a packed column.
Aroonwilas A and Veawab A
Industrial & Engineering Chemistry Research, 43(9), 2228-2237 (2004)
Modeling of CO2 capture by three typical amine solutions in hollow fiber membrane contactors.
Wang R, et al.
Chemical Engineering and Processing, 43(7), 849-856 (2004)
Selective absorption of H2S from gas streams containing H2S and CO2 into aqueous solutions of N-methyldiethanolamine and 2-amino-2-methyl-1-propanol.
Mandal BP, et al.
Separation and Purification Technology, 35(3), 191-202 (2004)
Hidetaka Yamada et al.
The journal of physical chemistry. A, 115(14), 3079-3086 (2011-03-19)
We used density functional theory (DFT) calculations with the latest continuum solvation model (SMD/IEF-PCM) to determine the mechanism of CO(2) absorption into aqueous solutions of 2-amino-2-methyl-1-propanol (AMP). Possible absorption process reactions were investigated by transition-state optimization and intrinsic reaction coordinate
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service