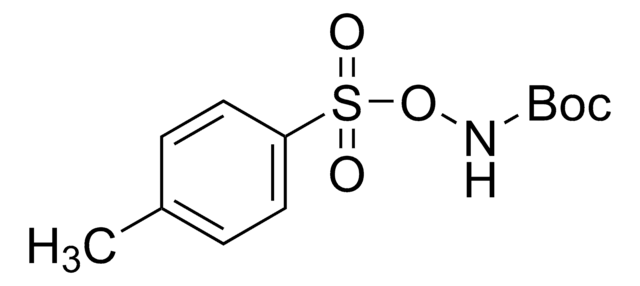
922722
O-Pivaloylhydroxyamine triflic acid
Synonym(s):
2,2-dimethyl-, azanyl ester, 1,1,1-trifluoromethanesulfonate, PivONH3OTF, Propanoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H11NO2 · CHF3O3S
CAS Number:
Molecular Weight:
267.22
UNSPSC Code:
12352106
NACRES:
NA.22
Recommended Products
form
solid
Quality Level
Application
This hydroxylamine-based reagent enables the iron-catalyzed selective transformation of thiols to sulfinamides under mild conditions without the use of precious metal catalysts or additional oxidants. This reagent is shelf stable and facilitates the reaction in a single step and under mild conditions.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Sayanti Chatterjee et al.
Angewandte Chemie (International ed. in English), 60(2), 758-765 (2020-09-22)
An iron catalyzed reaction for the selective transformation of thiols (-SH) to sulfinamides (-SONH2 ) by a direct transfer of -O and free -NH2 groups has been developed. The reaction operates under mild conditions using a bench stable hydroxylamine derived
Eric Falk et al.
Organic letters, 23(4), 1422-1426 (2021-02-06)
We report both an intermolecular C-H amination of arenes to access N-methylanilines and an intramolecular variant for the synthesis of tetrahydroquinolines. A newly developed, highly electrophilic aminating reagent was key for the direct synthesis of unprotected N-methylanilines from simple arenes.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service