All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C4HCl2FN2
CAS Number:
Molecular Weight:
166.97
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
37-41 °C (lit.)
SMILES string
Fc1cnc(Cl)nc1Cl
InChI
1S/C4HCl2FN2/c5-3-2(7)1-8-4(6)9-3/h1H
InChI key
WHPFEQUEHBULBW-UHFFFAOYSA-N
Application
2,4-Dichloro-5-fluoropyrimidine can be used as a starting material to synthesize:
- 5-fluoropyrimidine-2-carboxamides and 5-fluoropyrimidine-4-carboxamides as potential kinase inhibitors.
- A series of 2,4-diamino-5-fluoropyrimidine derivatives as potential protein kinase Cθ inhibitors.
- 2,4-Bisanilinopyrimidine derivatives as potential aurora kinases inhibitors.
- 5-fluoro-N,N-bis(4-methoxyphenyl)-2,4-pyrimidinediamine by reacting with p-methoxy aniline in the presence of DIPEA.
- 2-chloro-5-fluoro-4-(4-fluorophenyl)pyrimidine by Suzuki coupling reaction in the presence of (4-fluorophenyl)boronic acid triphenylphosphine, and palladium(II) acetate catalyst.
- 5-fluoro-2-(piperidin-4-yloxy)pyrimidin-4-amine, a scaffold, which is used in the preparation of potent deoxycytidine kinase inhibitors.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B - Skin Sens. 1A
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
222.8 °F - closed cup
Flash Point(C)
106 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Optimization of 2, 4-diamino-5-fluoropyrimidine derivatives as protein kinase C theta inhibitors with mitigated time-dependent drug-drug interactions and P-gp liability
Kunikawa S, et al.
Bioorganic & Medicinal Chemistry, 23(13), 3269-3277 (2015)
Design, synthesis, and biological evaluation of a series of novel AXL kinase inhibitors
Mollard A, et al.
ACS Medicinal Chemistry Letters, 2(12), 907-912 (2011)
Practical synthesis of 5-fluoro-2-(piperidin-4-yloxy) pyrimidin-4-amine, a key intermediate in the preparation of potent deoxycytidine kinase inhibitors
Zhang H, et al.
Organic Process Research & Development, 13(4), 807-811 (2009)
Ignacio Aliagas-Martin et al.
Journal of medicinal chemistry, 52(10), 3300-3307 (2009-05-01)
The two major Aurora kinases carry out critical functions at distinct mitotic stages. Selective inhibitors of these kinases, as well as pan-Aurora inhibitors, show antitumor efficacy and are now under clinical investigation. However, the ATP-binding sites of Aurora A and
Facile and regioselective synthesis of novel 2, 4-disubstituted-5-fluoropyrimidines as potential kinase inhibitors
Wada H, et al.
Tetrahedron Letters, 53(14), 1720-1724 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service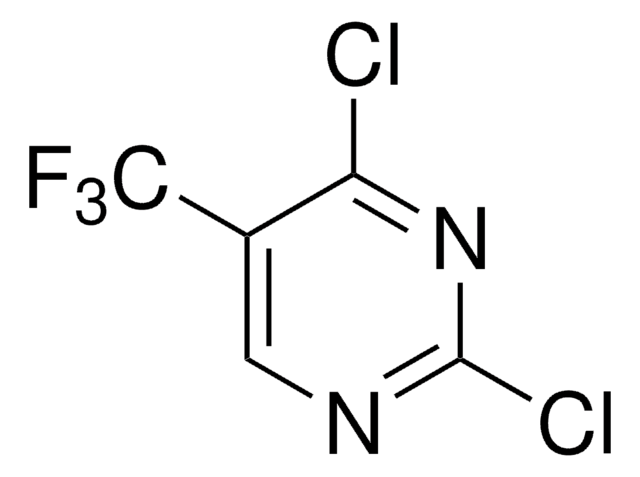
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)