All Photos(1)
About This Item
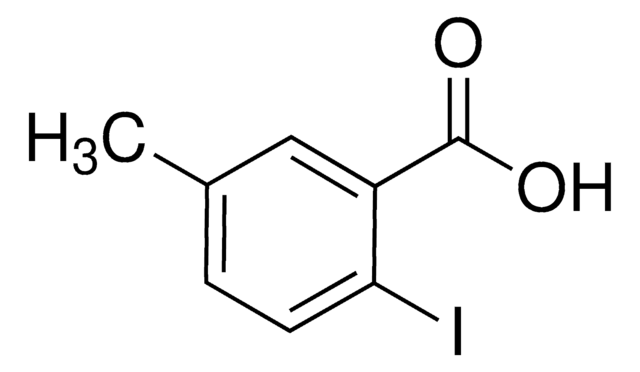
Linear Formula:
IC6H3(CH3)CO2H
CAS Number:
Molecular Weight:
262.04
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
97%
mp
150-153 °C (lit.)
SMILES string
Cc1cccc(C(O)=O)c1I
InChI
1S/C8H7IO2/c1-5-3-2-4-6(7(5)9)8(10)11/h2-4H,1H3,(H,10,11)
InChI key
PZUXUOZSSYKAMX-UHFFFAOYSA-N
Application
2-Iodo-3-methylbenzoic acid may be used in the synthesis of:
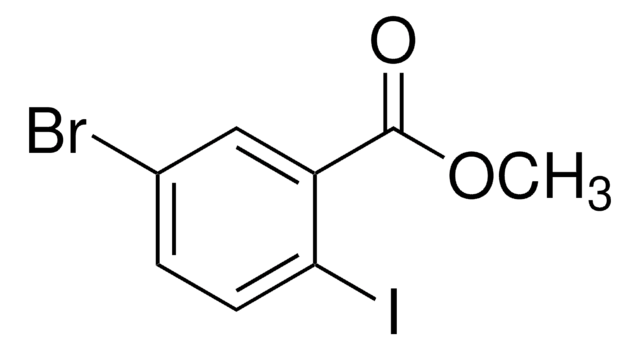
- methyl 2-iodo-3-methylbenzoate
- 10,11-dihydro-10-oxodibenz[b,f]oxepin-4-acetic acid
- 5-methyl-3-pentyl-1H-isochromen-1-one
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Asymmetric allylic oxidation with biarylbisoxazoline-copper (I) catalysis.
Andrus MB and Asgari D.
Tetrahedron, 56(32), 5775-5780 (2000)
Veronika Hampl et al.
Scientia pharmaceutica, 79(1), 21-30 (2011-05-28)
New isocoumarins were prepared in an efficient way from 2-iodobenzoic acid derivatives and hept-1-yne in a Sonogashira reaction, followed by spontaneous cyclization. Catalytic hydrogenation gave the corresponding dihydroisocoumarins. A 4-chloroisocoumarin was prepared on an alternative pathway. Some of the new
G W Rewcastle et al.
Journal of medicinal chemistry, 34(2), 491-496 (1991-02-01)
A series of tricyclic analogues of 9-oxo-9H-xanthene-4-acetic acid have been prepared and evaluated for their ability to cause hemorrhagic necrosis in subcutaneously implanted colon 38 tumors in mice, in an effort to extend the structure-activity relationships for this series. As
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service