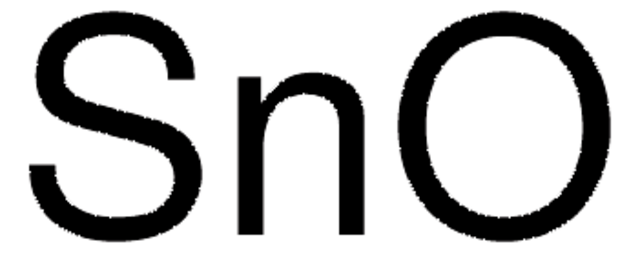549657
Tin(IV) oxide
nanopowder, ≤100 nm avg. part. size
Synonym(s):
Tin oxide, Stannic oxide
About This Item
Recommended Products
form
nanopowder
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
avg. part. size
≤100 nm
density
6.95 g/mL at 25 °C (lit.)
application(s)
battery manufacturing
greener alternative category
SMILES string
O=[Sn]=O
InChI
1S/2O.Sn
InChI key
XOLBLPGZBRYERU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
Storage Class Code
11 - Combustible Solids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Synthesis, Properties, and Applications of Perovskite-Phase Metal Oxide Nanostructures
Nanomaterials for Energy Storage in Lithium-ion Battery Applications
HEVs address rising fuel costs and emissions concerns, utilizing battery packs alongside internal combustion engines for enhanced performance.
Recent demand for electric and hybrid vehicles, coupled with a reduction in prices, has caused lithium-ion batteries (LIBs) to become an increasingly popular form of rechargeable battery technology.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service