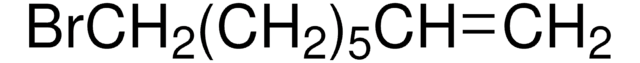All Photos(3)
About This Item
Linear Formula:
Br(CH2)5CH=CH2
CAS Number:
Molecular Weight:
177.08
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97%
refractive index
n20/D 1.466 (lit.)
bp
27 °C/1 mmHg (lit.)
density
1.162 g/mL at 25 °C (lit.)
SMILES string
BrCCCCCC=C
InChI
1S/C7H13Br/c1-2-3-4-5-6-7-8/h2H,1,3-7H2
InChI key
GNYDYUQVALBGGZ-UHFFFAOYSA-N
General description
7-Bromo-1-heptene is a linear halogenated alkene.
Application
7-Bromo-1-heptene may be used to synthesize:
- boc-L-2-amino-8-nonenoic acid (boc = tert-butyloxycarbonyl)
- 9-hept-6-enyloxy-5-hydroxymethyl-2-isopropyl-1-methyl-1,4,5,6-tetrahydro-2H-benzo[e][1,4] diazocin-3-one
- 8-hydroxy-15,15,15-trifluoro-1-pentadece
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
136.9 °F - closed cup
Flash Point(C)
58.3 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Alan P Kozikowski et al.
ChemMedChem, 4(7), 1095-1105 (2009-04-28)
A series of benzolactam compounds were synthesized, some of which caused a concentration-dependent increase in sAPPalpha and decrease in Abeta production in the concentration range of 0.1-10 microM. Moreover, some compounds showed neuroprotective effects in the 10-20 microM range in
G H Stoll et al.
Journal of lipid research, 32(5), 843-857 (1991-05-01)
An analogue of the long-chain fatty acid salt, sodium stearate, was synthesized in which the hydrogen atoms at carbons 2, 3, and 18 were replaced by fluorine. The key step in the synthesis was the addition of 3-iodo-2,2,3,3-tetrafluoropropanoic acid amide
Norikazu Nishino et al.
Bioorganic & medicinal chemistry, 16(1), 437-445 (2007-09-29)
Inhibitors of histone deacetylases (HDACs) are a promising class of anticancer agents that effect gene regulation. To know the interaction of aliphatic cap groups with HDACs, cyclic tetrapeptide and bicyclic peptide disulfide hybrids were synthesized without aromatic ring in their
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service