All Photos(1)
About This Item
Empirical Formula (Hill Notation):
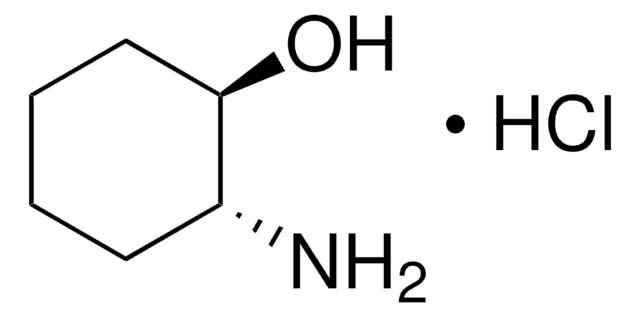
C5H11NO · HCl
CAS Number:
Molecular Weight:
137.61
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
191-196 °C (lit.)
functional group
hydroxyl
SMILES string
Cl.N[C@H]1CCC[C@@H]1O
InChI
1S/C5H11NO.ClH/c6-4-2-1-3-5(4)7;/h4-5,7H,1-3,6H2;1H/t4-,5-;/m0./s1
InChI key
ZFSXKSSWYSZPGQ-FHAQVOQBSA-N
General description
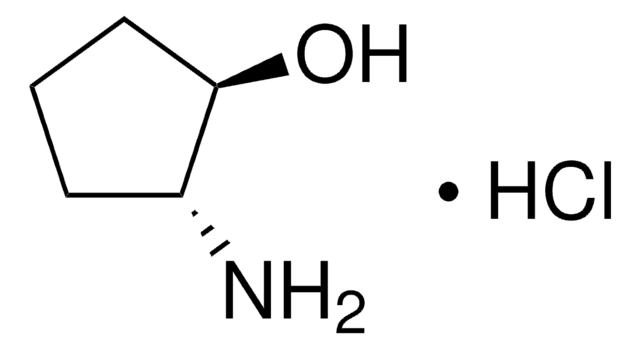
trans-2-Aminocyclopentanol hydrochloride is an aminocyclanol. Its d,l-cis- and d,l-trans- forms have been synthesized. The trans-form of the product can be produced in large (multigram) scale via carbamate addition protocol. Cholinesterase inhibitory potential of cis-form of 2-aminocyclopentanol hydrochloride is higher (twofold) that of its trans-form.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
James A Birrell et al.
Organic letters, 15(12), 2895-2897 (2013-06-08)
A highly enantioselective addition of phenyl carbamate to meso-epoxides has been developed to efficiently generate protected trans-1,2-amino alcohols. This transformation is promoted by an oligomeric (salen)Co-OTf catalyst and has been used to prepare two useful 2-aminocycloalkanol hydrochlorides in enantiopure form
Preparation of antidotes for anticholinesterase poisoning. IV. Synthesis and protective effectiveness of 2'-(cis-and trans-2'-hydroxycyclohexyl) aminoethyl 1-phenylcyclopentanecarboxylate hydrochlorides.
Bannard RAB and Parkkari JH.
Canadian Journal of Chemistry, 48(9), 1377-1382 (1970)
Stereochemistry of Aminocyclanols. Synthesis of cis Epimers via Oxazolines. The 2-Aminocyclopentanols*.
McCasland GE and Smith DA.
Journal of the American Chemical Society, 72(5), 2190-2195 (1950)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service