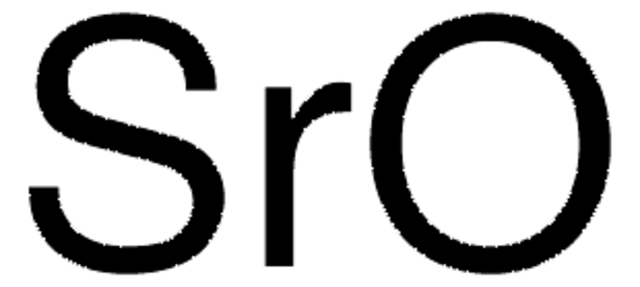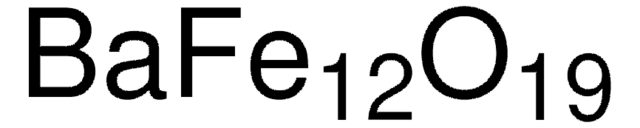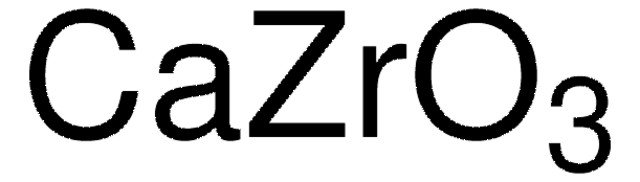517011
Strontium titanate
nanopowder, <100 nm particle size, 99% trace metals basis
Synonym(s):
Strontium metatitanate, Strontium titanium trioxide
About This Item
Recommended Products
Quality Level
Assay
99% trace metals basis
form
nanopowder
dielectric constant
300
reaction suitability
reagent type: catalyst
core: titanium
particle size
<100 nm
mp
2060 °C (lit.)
density
4.81 g/mL at 25 °C (lit.)
SMILES string
[Sr++].[O-][Ti]([O-])=O
InChI
1S/3O.Sr.Ti/q;2*-1;+2;
InChI key
VEALVRVVWBQVSL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Photoinduced electronic and ionic effects in strontium titanate: Focuses on the interaction of strontium titanate with ultraviolet radiation, investigating photoionic processes and photochromic effects, which are crucial for developing optoelectronic devices (M Siebenhofer et al., 2021).
- The emerging career of strontium titanates in photocatalytic applications: Reviews the role of strontium titanates in photocatalytic applications, particularly emphasizing their utility in environmental remediation processes (N Sharma, K Hernadi, 2022).
- Recent advances on carrier and exciton self-trapping in strontium titanate: Discusses the self-trapping of carriers and excitons in strontium titanate, providing insights into its electronic properties and implications for semiconductor technologies (ML Crespillo et al., 2019).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Synthesis, Properties, and Applications of Perovskite-Phase Metal Oxide Nanostructures
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service