Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
195529
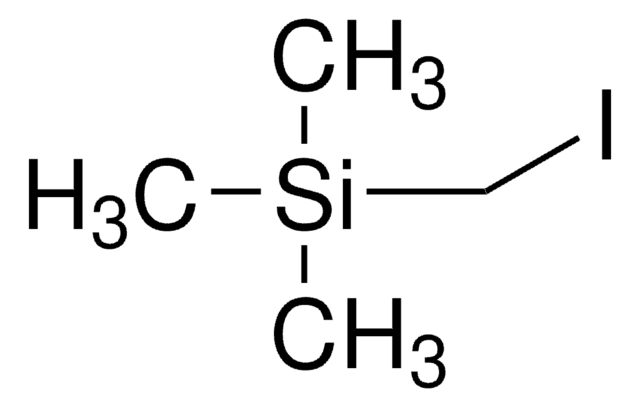
Iodotrimethylsilane
97%
Synonym(s):
TMIS, Trimethyliodosilane
Select a Size
Select a Size
About This Item
Recommended Products
Quality Level
Assay
97%
form
liquid
contains
copper as stabilizer
refractive index
n20/D 1.471 (lit.)
bp
106 °C (lit.)
density
1.406 g/mL at 25 °C (lit.)
storage temp.
−20°C
SMILES string
C[Si](C)(C)I
InChI
1S/C3H9ISi/c1-5(2,3)4/h1-3H3
InChI key
CSRZQMIRAZTJOY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
[1]Iodotrimethylsilane is a hard-soft reagent that reacts readily with organic compounds containing oxygen (a hard base) forming a strong siliconoxygen bond.[2]
Application
accessory
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-23.8 °F - closed cup
Flash Point(C)
-31 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
-
What is the Department of Transportation shipping information for this product?
1 answer-
Helpful?
-
-
What is known of the mechanism of reactions involving product 195529, Iodotrimethylsilane?
1 answer-
The mechanism for cleavage of esters is described in PNAS, 75(1), 4-6 (1978).The authors go on to explain that the data do not support a previously published mechanism via a carbenium iodide.The paper also discusses the cleavage of ethers using iodotrimethylsilane. It appears that this is not as efficient as cleavage of esters, and a couple of mechanisms (pages 5 and 6) are proposed.Olah also described the cleavage of the phosphate esters in peptides that contain phosphate esters, using iodotrimethylsilane (Tetrahedron, 38, 2225 (1982)). The amide linkages in the peptide backbone are not cleaved.
Helpful?
-
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service