All Photos(2)
About This Item
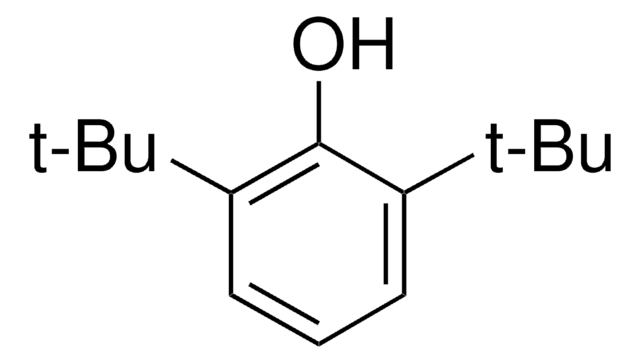
Linear Formula:
(CH3)3CC6H4OH
CAS Number:
Molecular Weight:
150.22
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
bp
125-130 °C/20 mmHg (lit.)
mp
44-46 °C (lit.)
SMILES string
CC(C)(C)c1cccc(O)c1
InChI
1S/C10H14O/c1-10(2,3)8-5-4-6-9(11)7-8/h4-7,11H,1-3H3
InChI key
CYEKUDPFXBLGHH-UHFFFAOYSA-N
Gene Information
mouse ... Esr1(13982)
Related Categories
General description
3-tert-Butylphenol undergoes stereoselective hydrogenation over charcoal-supported rhodium catalyst in supercritical carbon dioxide solvent.
Application
3-tert-Butylphenol has been used to study the effect of alkyl group on the phenol ring on the estrogenic potency of alkylphenolic compounds in the yeast screen.
Other Notes
Contains 4-tert-butylphenol
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
228.2 °F - closed cup
Flash Point(C)
109 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Stereoselective hydrogenation of tert-butylphenols over charcoal-supported rhodium catalyst in supercritical carbon dioxide solvent.
Hiyoshi N, et al.
J. Catal., 252(1), 57-68 (2007)
Tadashi Toyama et al.
Biodegradation, 21(2), 157-165 (2009-08-26)
A novel bacterium capable of utilizing 2-sec-butylphenol as the sole carbon and energy source, Pseudomonas sp. strain MS-1, was isolated from freshwater sediment. Within 30 h, strain MS-1 completely degraded 1.5 mM 2-sec-butylphenol in basal salt medium, with concomitant cell
Cynthia D Selassie et al.
Journal of medicinal chemistry, 48(23), 7234-7242 (2005-11-11)
In this comprehensive study on the caspase-mediated apoptosis-inducing effect of 51 substituted phenols in a murine leukemia cell line (L1210), we determined the concentrations needed to induce caspase activity by 50% (I50) and utilized these data to develop the following
Alfonso Pérez-Garrido et al.
Bioorganic & medicinal chemistry, 17(2), 896-904 (2008-12-06)
This paper reports a QSAR study for predicting the complexation of a large and heterogeneous variety of substances (233 organic compounds) with beta-cyclodextrins (beta-CDs). Several different theoretical molecular descriptors, calculated solely from the molecular structure of the compounds under investigation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service