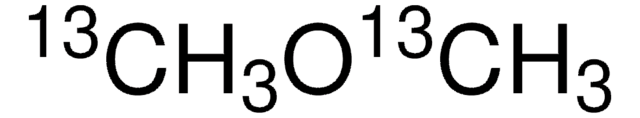38912
Dimethyl ether
puriss., ≥99.9% (GC)
Synonym(s):
Methyl ether
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2O
CAS Number:
Molecular Weight:
46.07
Beilstein:
1730743
EC Number:
MDL number:
UNSPSC Code:
12142100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
1.62 (vs air)
vapor pressure
>760 mmHg ( 25 °C)
grade
puriss.
Assay
≥99.9% (GC)
autoignition temp.
662 °F
expl. lim.
27 %
bp
−24.8 °C (lit.)
mp
−141 °C (lit.)
SMILES string
COC
InChI
1S/C2H6O/c1-3-2/h1-2H3
InChI key
LCGLNKUTAGEVQW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Dimethyl ether (DME) is the simplest aliphatic ether used as a reactant as well as a solvent in organic synthesis. It is also used as a precursor for the preparation of important useful organic compounds such as dimethyl carbonate, methyl acetate, propylene and chloromethyl ether.
Application
DME can be used as a precursor for the preparation of versatile methylating agents such as dimethyl sulfate and trimethyloxonium tetrafluoroborate.
Packaging
Cylinder with net 1.1 kg
DIN 477 nr.1
Other Notes
Sales restrictions may apply
Recommended products
Z742161 DIN 1 Regulator OR 99112 outlet Valve
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Gas 1A - Press. Gas Liquefied gas
Storage Class Code
2A - Gases
WGK
WGK 1
Flash Point(F)
-41.8 °F - closed cup
Flash Point(C)
-41 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Trimethyloxonium tetrafluoroborate
Curphey, TJ
Organic Syntheses, 50(4), 1019-1023 (1988)
Dimethyl ether
Muller M, et al.
Ullmann's Encyclopedia of Industrial Chemistry, 156(2), 497-511 (2000)
Dimethyl ether (DME) as an alternative fuel
Semelsberger TA, et al.
Journal of Power Sources, 156(2), 497-511 (2006)
Direct dimethyl ether synthesis
Ogawa T, et al.
Journal of natural gas chemistry, 12(4), 219-227 (2003)
Wu Zhou et al.
Nature chemistry, 1(9), 722-728 (2010-12-03)
Tungstated zirconia is a robust solid acid catalyst for light alkane (C(4)-C(8)) isomerization. Several structural models for catalytically active sites have been proposed, but the topic remains controversial, partly because of the absence of direct structural imaging information on the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service