ROAPRO
Roche
Aprotinin
from bovine lung
Synonym(s):
Aprotinin, pancreatic trypsin inhibitor, trypsin inhibitor, pancreas type (bpti), trypsin-kallikrein inhibitor
About This Item
Recommended Products
biological source
bovine lung
Quality Level
form
lyophilized
packaging
pkg of 10 mg (10236624001)
pkg of 100 mg (11583794001)
pkg of 50 mg (10981532001)
manufacturer/tradename
Roche
technique(s)
electrophoresis: suitable
tissue culture: suitable
pH range
3-10
solubility
water: soluble 10 mg/mL
absorption
0.84 at 280 nm
shipped in
wet ice
storage temp.
2-8°C
Related Categories
General description
Specificity
Cathepsin G, acrosin, human leukocyte elastase, and human urokinase are weakly inhibited. Factor Xa, thrombin, subtilisin, papain, pepsin, angiotensin-converting enzyme (ACE), carboxypeptidase A and B, other metalloproteases, and thiolproteases are not inhibited.
Application
- Further applications: Purification of urokinase, trypsin, and chymotrypsin on immobilized aprotinin
- Quantification of kallikrein activity in mixtures of esterases and proteases
- Controlled degradation of substrates by avoiding nonspecific proteolysis in clinical chemical tests
- Aprotinin as a model protein in protein-folding studies
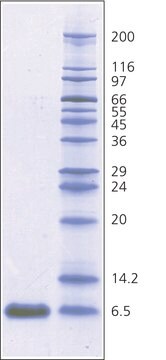
- Molecular weight marker in SDS-polyacrylamide gel electrophoresis
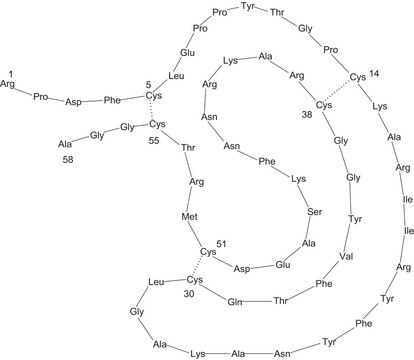
Sequence
Unit Definition
One inhibitor unit (IU) (+25 °C, BAEE as substrate) corresponds to about 2.8 inhibitor units (+25 °C, Chromozym TRY as substrate).
One inhibitor unit (IU) (+25 °C, BAEE as substrate) corresponds to about 26 kallikrein inhibitor units (KIU) (+25 °C).
One inhibitor unit (IU) (+25 °C, BAEE as substrate) corresponds to about 0.067 inhibitor units (+25 °C; Bz-D,L-Arg-4-Na as substrate, trypsin determination at pH 7.8).
One kallikrein inhibitor unit = 0.17 μg crystalline aprotinin.
Preparation Note
Working solution: Soluble in water (10 mg/ml) or aqueous buffer solution (e.g., 0.1 M Tris, pH 8.0).
Note: To avoid adsorption of aprotinin onto negatively charged solid phases, e.g., chromatography gels, ultrafiltration membranes, the NaCl concentration should be above 0.1 M or other suitable salts should be added to all buffers used during the separation.
Storage conditions (working solution): -15 to -25 °C
Reconstitution
Aliquots stored at -15 to -25 °C are stable for approximately 6 months.
Note: Avoid repeated freezing and thawing and exposure to strongly alkaline solutions (inactive at pH > 12.8).
Other Notes
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
fractional diagonal chromatography
Kinase and Phosphatidylinositide 3-Kinase/Akt Signaling
Articles
While aprotinin and bovine pancreatic trypsin inhibitor (BPTI) are the same protein sequence, the term aprotinin is typically used when describing the protein derived from bovine lung.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service