QBD10273
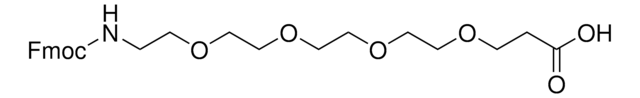
Fmoc-N-amido-dPEG®8-acid
>95% (HPLC)
Synonym(s):
Fmoc-N-amido-PEG8-COOH, Fmoc-N-amido-PEG8-acid, Fmoc-NH-PEG8-acid, Fmoc-PEG-acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C34H49NO12
Molecular Weight:
663.75
MDL number:
UNSPSC Code:
12352106
NACRES:
NA.22
Recommended Products
Assay
>95% (HPLC)
form
solid or viscous liquid
reaction suitability
reaction type: Pegylations
polymer architecture
shape: linear
functionality: heterobifunctional
shipped in
ambient
storage temp.
−20°C
Features and Benefits
Fmoc-N-amido-dPEG8-acid is a monodisperse PEG product that is useful for peptide synthesis. The 28-atom dPEG spacer allows the introduction of a medium-length, hydrophilic spacer onto either end of a peptide chain or between two peptide chains. The flexible dPEG spacer conjugates to peptides using conventional peptide synthesis chemistry. Peptide PEGylation imparts water solubility to hydrophobic peptide chains. Also, PEGylated peptides have expanded hydrodynamic volumes, which can reduce or eliminate renal clearance, and are protected from proteolysis. The combination of decreased renal clearance and protection from proteolysis contributes to longer in vivo circulation times for PEGylated (as compared to non-PEGylated) peptides. Additionally, PEGylation diminishes a peptide′s antigenicity. This product is part of the Fmoc-N-amido-dPEGn-acid (n=2, 3, 4, 5, 6, 8, 12, 24, 36) product series.
Legal Information
Products Protected under U.S. Patent #s 7,888,536 & 8,637,711 and European Patent #s 1,594,440 & 2,750,681
dPEG is a registered trademark of Quanta BioDesign
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Guangchang Zhou et al.
Molecular bioSystems, 8(9), 2395-2404 (2012-07-10)
Robust methods for highly parallel, quantitative analysis of cellular protein tyrosine kinase activities may provide tools critically needed to decipher oncogenic signaling, discover new targeted drugs, diagnose cancer and monitor patients. Here, we describe proof-of-principle for a novel protein kinase
Ignacio Melgar-Asensio et al.
Investigative ophthalmology & visual science, 59(10), 4071-4081 (2018-08-12)
Drug delivery by intravitreal injection remains problematic, small agents and macromolecules both clearing rapidly. Typical carriers use microparticles (>2 μm), with size-related liabilities, to slow diffusion. We recently described cationic nanoparticles (NP) where conjugated Arg peptides prolonged residence in rat
Ian W Hamley
Biomacromolecules, 15(5), 1543-1559 (2014-04-12)
The remarkable diversity of the self-assembly behavior of PEG-peptides is reviewed, including self-assemblies formed by PEG-peptides with β-sheet and α-helical (coiled-coil) peptide sequences. The modes of self-assembly in solution and in the solid state are discussed. Additionally, applications in bionanotechnology
Jared F Stefanick et al.
ACS nano, 7(9), 8115-8127 (2013-09-06)
Ligand-targeted nanoparticles are emerging drug delivery vehicles for cancer therapy. Here, we demonstrate that the cellular uptake of peptide-targeted liposomes and micelles can be significantly enhanced by increasing the hydrophilicity of the targeting peptide sequence while simultaneously optimizing the EG
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service