GEN-7003
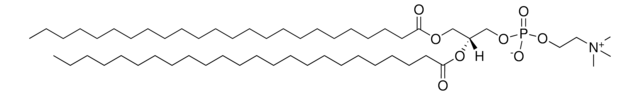
Cationic Liposomes for DNA/RNA delivery
DOTAP:Cholesterol (1:1) containing 0.5% NBD-DOPE (Fluorescent)
About This Item
Recommended Products
Quality Level
contains
NBD-DOPE (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(7-nitro-2-
1,3-benzoxadiazol-4-yl)) as fluorescent label
composition
Deionized RNAse-free Water
concentration
0.02 mM (NBD-DOPE)
2 mM (Cholesterol)
2 mM (DOTAP)
impurities
1:1 mol/mol DOTAP:Cholesterol
particle size
100 nm
pH
7
absorbance ratio
460/535 nm
Looking for similar products? Visit Product Comparison Guide
General description
Application
Gene delivery
Lipid-protein interactions
Storage and Stability
Liposomes are made under sterile conditions. If you need to take multiple aliquots out of the vial, it is advised to take extreme care in not contaminating the vial. It is recommended to handle the vial under a sterile hood to maintain the sterility of the product. Liposomes should never be frozen. Ice crystals that form during freezing will rupture the lipid membrane of the liposomes and change the size of liposomes particles.
Legal Information
Disclaimer
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
LNPs are ideal carriers for mRNA drugs, as evident from the two FDA-approved SARS-CoV-2 vaccines. However, efficient LNPs need further research on ionizable lipid selection, formulation, and administration. This review examines lipid usage, ionizable lipid selection, and LNP design for mRNA drug delivery.
LNPs are ideal carriers for mRNA drugs, as evident from the two FDA-approved SARS-CoV-2 vaccines. However, efficient LNPs need further research on ionizable lipid selection, formulation, and administration. This review examines lipid usage, ionizable lipid selection, and LNP design for mRNA drug delivery.
LNPs are ideal carriers for mRNA drugs, as evident from the two FDA-approved SARS-CoV-2 vaccines. However, efficient LNPs need further research on ionizable lipid selection, formulation, and administration. This review examines lipid usage, ionizable lipid selection, and LNP design for mRNA drug delivery.
LNPs are ideal carriers for mRNA drugs, as evident from the two FDA-approved SARS-CoV-2 vaccines. However, efficient LNPs need further research on ionizable lipid selection, formulation, and administration. This review examines lipid usage, ionizable lipid selection, and LNP design for mRNA drug delivery.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service