A12658
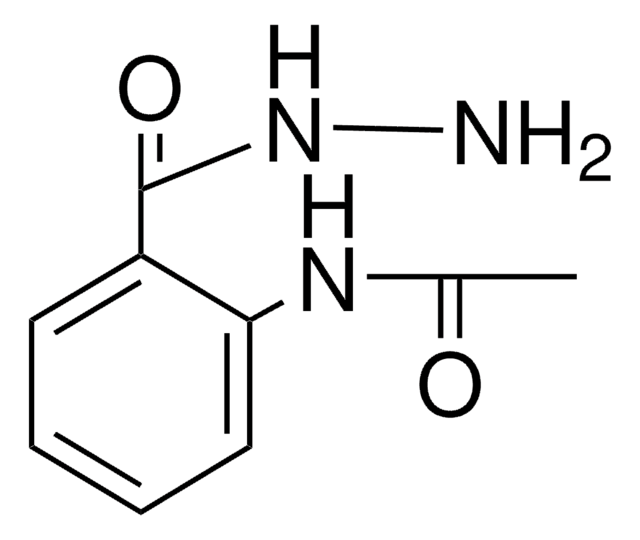
N-Acetylanthranilic acid
99%
Synonym(s):
2-Acetamidobenzoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3CONH)C6H4CO2H
CAS Number:
Molecular Weight:
179.17
Beilstein:
880371
EC Number:
MDL number:
UNSPSC Code:
12352200
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
reaction suitability
reaction type: solution phase peptide synthesis
color
off-white to tan
mp
184-187 °C (lit.)
application(s)
peptide synthesis
SMILES string
CC(=O)Nc1ccccc1C(O)=O
InChI
1S/C9H9NO3/c1-6(11)10-8-5-3-2-4-7(8)9(12)13/h2-5H,1H3,(H,10,11)(H,12,13)
InChI key
QSACCXVHEVWNMX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Nikolai E Polyakov et al.
Organic & biomolecular chemistry, 3(5), 881-885 (2005-02-26)
CIDNP techniques were applied to the investigation of the elementary mechanism of photoinduced interaction between anti-arrhythmic drug lappaconitine and amino acids tyrosine and tryptophan. It has been shown that the reactions involve the formation of lappaconitine radical anion. Lappaconitine radical
H K Hund et al.
Biological chemistry Hoppe-Seyler, 371(10), 1005-1008 (1990-10-01)
Quinaldine catabolism was investigated with the bacterial strain Arthrobacter sp., which is able to grow aerobically in a mineral salt medium with quinaldine as sole source of carbon, nitrogen and energy. The following degradation products of quinaldine were isolated from
Stephan Kolkenbrock et al.
Journal of bacteriology, 188(24), 8430-8440 (2006-10-17)
N-acetylanthranilate amidase (Amq), a 32.8-kDa monomeric amide hydrolase, is involved in quinaldine degradation by Arthrobacter nitroguajacolicus Rü61a. Sequence analysis and secondary structure predictions indicated that Amq is related to carboxylesterases and belongs to the alpha/beta-hydrolase-fold superfamily of enzymes; inactivation of
Jörg Overhage et al.
Microbiology (Reading, England), 151(Pt 2), 491-500 (2005-02-09)
Arthrobacter nitroguajacolicus Rü61a, which utilizes quinaldine as sole source of carbon and energy, was shown to contain a conjugative linear plasmid of approximately 110 kb, named pAL1. It exhibits similarities with other linear plasmids from Actinomycetales in that it has
Christine Müller et al.
Applied and environmental microbiology, 80(23), 7266-7274 (2014-09-23)
A bacterial strain, which based on the sequences of its 16S rRNA, gyrB, catA, and qsdA genes, was identified as a Rhodococcus sp. closely related to Rhodococcus erythropolis, was isolated from soil by enrichment on the Pseudomonas quinolone signal [PQS;
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service