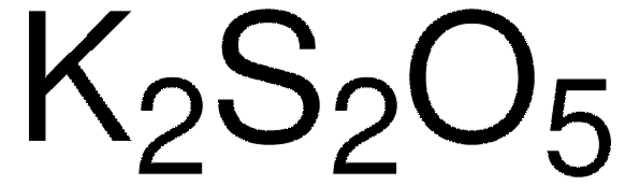658510
Potassium sulfite
90%
Synonym(s):
dipotassiumsulfite
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
K2SO3
CAS Number:
Molecular Weight:
158.26
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
90%
SMILES string
[K+].[K+].[O-]S([O-])=O
InChI
1S/2K.H2O3S/c;;1-4(2)3/h;;(H2,1,2,3)/q2*+1;/p-2
InChI key
BHZRJJOHZFYXTO-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Application
Potassium sulfite (K2SO3) can be used as a catalyst in the oxidation of sulfur dioxide. It is also used as an anionic catalyst for the cyclotrimerization of aryl isocyanates to prepare heterocyclic symmetrical isocyanurates.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
David Crich et al.
Journal of the American Chemical Society, 128(8), 2544-2545 (2006-02-24)
Alkylation of potassium selenosulfate with allylic halides gives Se-allyl seleno Bunte salts. On reaction with thiols at room temperature, these afford mixed dialkyl selenosulfides, which undergo 2,3-sigmatropic rearrangement with loss of selenium, either spontaneously or with assistance by triphenylphosphine, thereby
The reactivity of products of thermal interaction between ammonium meta-vanadate and potassium sulfite as catalysts for oxidation of sulfur dioxide
Fouda MFR, et al.
Applied Catalysis A: General, 223(1-2), 11-27 (2002)
Sulfate catalysed multicomponent cyclisation reaction of aryl isocyanates under green conditions
Dekamin, Mohammad G, et al.
J. Chem. Res. (M), 2005(3), 177-179 (2005)
Xinjian Shi et al.
Nature communications, 5, 4775-4775 (2014-09-03)
Tungsten trioxide/bismuth vanadate heterojunction is one of the best pairs for solar water splitting, but its photocurrent densities are insufficient. Here we investigate the advantages of using helical nanostructures in photoelectrochemical solar water splitting. A helical tungsten trioxide array is
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service