All Photos(1)
About This Item
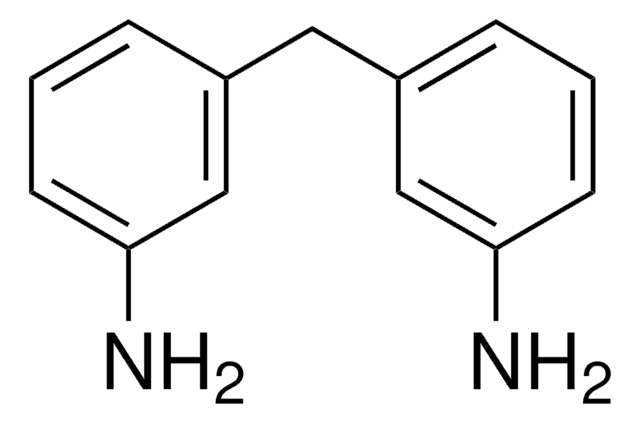
Linear Formula:
CH3CO2(CH2)4CH=CH2
CAS Number:
Molecular Weight:
142.20
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.423 (lit.)
bp
173-174 °C (lit.)
density
0.883 g/mL at 25 °C (lit.)
SMILES string
CC(=O)OCCCCC=C
InChI
1S/C8H14O2/c1-3-4-5-6-7-10-8(2)9/h3H,1,4-7H2,2H3
InChI key
MPLWNENKBSBMFN-UHFFFAOYSA-N
General description
5-Hexenyl acetate is a linear ester. It can undergo ruthenium-catalyzed cross-metathesis reactions with α-substituted vinyl boronates in dichloromethane. It participates in the post-polymerization modification step during the preparation of poly(vinylnorbornene).
Application
5-Hexenyl acetate may be used in the synthesis of (4E,7Z)-4,7-tridecadienyl acetate.
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
141.1 °F - closed cup
Flash Point(C)
60.6 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Xicotencatl Camacho-Coronel et al.
Frontiers in plant science, 11, 121-121 (2020-03-12)
Numerous plant-derived volatile organic compounds (VOCs) induce the expression of resistance-related genes and thereby cause an "associational resistance" in neighbouring plants. However, VOCs can also be sequestered by plant cuticular waxes. In case that they maintain their biological activity, such
Nonconjugated dienes from 1-alkenes: Application to the synthesis of sex pheromone (4E, 7Z)-4, 7-Tridecadienyl acetate
Kim TH and Park KM
Tetrahedron Letters, 36.27, 4833-4836 (1995)
Ring-opening metathesis polymerization of vinylnorbornene and following polymer modifications
Balcar H, et al.
Journal of Polymer Research, 21.9, 1-8 null
Synthesis of tri-substituted vinyl boronates via ruthenium-catalyzed olefin cross-metathesis
Morrill C, et al.
Tetrahedron Letters, 45.41 , 7733-7736 (2004)
Xuejiao Gao et al.
Nature communications, 9(1), 118-118 (2018-01-11)
It is known that self-assembled molecular monolayer doping technique has the advantages of forming ultra-shallow junctions and introducing minimal defects in semiconductors. In this paper, we report however the formation of carbon-related defects in the molecular monolayer-doped silicon as detected
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![(3R)-3-{[(9H-Fluoren-9-ylmethoxy)carbonyl]amino}-3-(3-methylphenyl)propanoic acid AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/369/030/c37536c8-fce7-456d-a3f2-1b29a57c2c52/640/c37536c8-fce7-456d-a3f2-1b29a57c2c52.png)