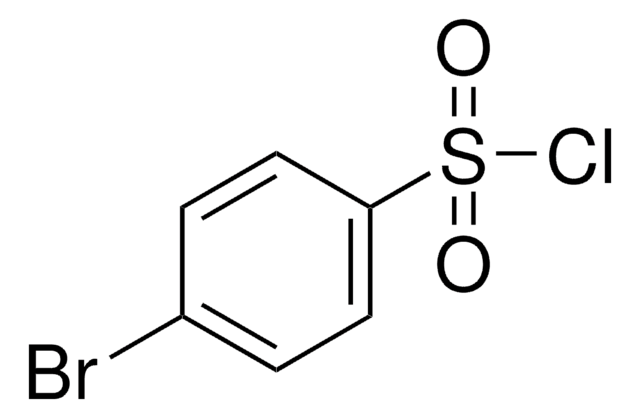
332925
4-Chlorobenzenesulfonic acid
technical grade, 90%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
ClC6H4SO3H
CAS Number:
Molecular Weight:
192.62
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Assay
90%
impurities
<3.5% H2SO4
bp
149 °C/22 mmHg (lit.)
SMILES string
OS(=O)(=O)c1ccc(Cl)cc1
InChI
1S/C6H5ClO3S/c7-5-1-3-6(4-2-5)11(8,9)10/h1-4H,(H,8,9,10)
InChI key
RJWBTWIBUIGANW-UHFFFAOYSA-N
General description
4-Chlorobenzenesulfonic acid is the major polar by-product formed during the chemical synthesis of 1,1,1-trichloro-2,2-bis-(4-chlorophenyl)ethane (DDT).
Application
4-Chlorobenzenesulfonic acid was employed as anion dopants for the matrix-assisted laser desorption/ionization-mass spectrometry (MALDI-MS) of neutral oligosaccharides.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A W Wong et al.
Analytical chemistry, 72(7), 1419-1425 (2000-04-14)
Alkylsulfonates are examined as anion dopants for the matrix-assisted laser desorption/ionization-mass spectrometry (MALDI-MS) of neutral oligosaccharides. The anion dopants allow neutral oligosaccharides to be examined in the same mixture as acidic oligosaccharides. The alkylsulfonate dopants interact strongly with the oligosaccharide
Rafael Blasco et al.
Environmental microbiology, 10(6), 1591-1600 (2008-03-12)
Pseudomonas aeruginosa RW41 is the first bacterial strain, which could be isolated by virtue of its capability to mineralize 4-chlorobenzenesulfonic acid (4CBSA), the major polar by-product of the chemical synthesis of 1,1,1-trichloro-2,2-bis-(4-chlorophenyl)ethane (DDT). This capability makes the isolate a promising
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service