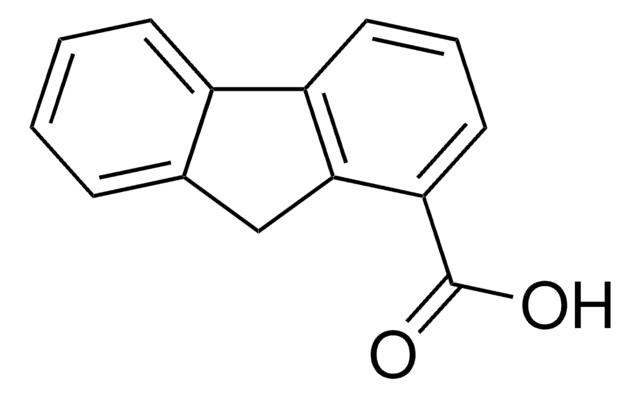
301663
9-Fluorenone-1-carboxylic acid
99%
Synonym(s):
9-Oxo-1-fluorenecarboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C14H8O3
CAS Number:
Molecular Weight:
224.21
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
mp
196-198 °C (lit.)
functional group
carboxylic acid
ketone
SMILES string
OC(=O)c1cccc2-c3ccccc3C(=O)c12
InChI
1S/C14H8O3/c15-13-10-5-2-1-4-8(10)9-6-3-7-11(12(9)13)14(16)17/h1-7H,(H,16,17)
InChI key
CBEFMGJHEKAMNI-UHFFFAOYSA-N
General description
9-Fluorenone-1-carboxylic acid is formed as an intermediate during four-ring polycyclic aromatic hydrocarbon fluoranthene degradation by Pseudomonas alcaligenes strain PA-10.
Application
9-Fluorenone-1-carboxylic acid was used in preparation of (E)- and (Z)-isomers of twisted 1,1′-dibromobifluorenylidene.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Hindy E Bronstein et al.
Journal of the American Chemical Society, 124(30), 8870-8875 (2002-07-26)
Diindeno[1,2,3,4-defg;1',2',3',4'-mnop]chrysene (1), the smallest possible alkene-centered C60 substructure with a curved pi-system, is obtained in 25-35% yield by flash vacuum pyrolysis of the twisted 1,1'-dibromobifluorenylidene (2) on a 100 mg scale at 1050 degrees C. At 1200 degrees C, the
L Gordon et al.
Biodegradation, 12(6), 393-400 (2002-06-08)
Pseudomonas alcaligenes strain PA-10 degrades the four-ring polycyclic aromatic hydrocarbon fluoranthene, co-metabolically. HPLC analysis of the growth medium identified four intermediates, 9-fluorenone-1-carboxylic acid; 9-hydroxy-1-fluorene carboxylic acid; 9-fluorenone and 9-fluorenol, formed during fluoranthene degradation. Pre-exposure of PA-10 to 9-fluorenone-1-carboxylic acid and
Jingnan Jin et al.
Environmental science and pollution research international, 24(1), 363-371 (2016-10-11)
In this study, a gram-positive fluoranthene-degrading bacterial strain was isolated from crude oil in Dagang Oilfield and identified as Microbacterium paraoxydans JPM1 by the analysis of 16S rDNA sequence. After 25 days of incubation, the strain JPM1 could degrade 91.78 % of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service