159425
9-Chloroacridine
97%
Synonym(s):
9-Chloroacridine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C13H8ClN
CAS Number:
Molecular Weight:
213.66
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
116-120 °C (lit.)
SMILES string
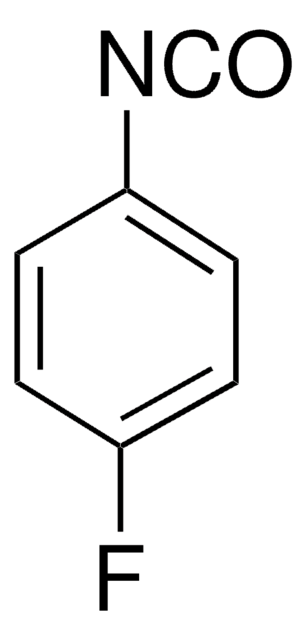
Clc1c2ccccc2nc3ccccc13
InChI
1S/C13H8ClN/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1-8H
InChI key
BPXINCHFOLVVSG-UHFFFAOYSA-N
Application
9-Chloroacridine was employed as chromogenic reagent in the spectrophotometric method for the quantitative determination of dapsone. It was also used in the synthesis of:
- series of novel chalcones bearing acridine moiety attached to the amino group in their ring A
- new acridine derivatives
- 9-phenoxyacridine and 4-phenoxyfuro[2,3-b]quinoline derivatives
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Paula Bosch et al.
Materials (Basel, Switzerland), 12(18) (2019-09-22)
A second-generation poly(propylene imine) dendrimer modified with acridine and its Cu(II) complex have been synthesized for the first time. It has been found that two copper ions form complexes with the nitrogen atoms of the dendrimeric core by coordinate bonds.
I Shoukrallah et al.
Die Pharmazie, 45(9), 675-677 (1990-09-01)
A spectrophotometric method for the quantitative determination of dapsone (1) has been developed through a condensation reaction of 9-chloroacridine as a chromogen and the amino groups of 1. The reaction variables were investigated and optimized. The resultant colored products is
V Tomar et al.
European journal of medicinal chemistry, 45(2), 745-751 (2009-12-22)
A series of novel chalcones bearing acridine moiety attached to the amino group in their ring A have been synthesized through noncatalyzed nucleophilic aromatic substitution reaction between various 3'-aminochalcone or 4'-aminochalcones and 9-chloroacridine. The synthesized chalcone derivatives have been characterized
Arumugasamy Elangovan et al.
Organic & biomolecular chemistry, 2(21), 3113-3118 (2004-10-27)
Electrogenerated chemiluminescence (ECL) of six new ethyne-based acridine derivatives (1-6) has been studied. The new acridine derivatives were synthesized by cross-coupling of 9-chloroacridine and corresponding donor-substituted phenylethynes under modified Sonogashira conditions. The donor groups were varied in the order of
Yeh-Long Chen et al.
Bioorganic & medicinal chemistry, 11(18), 3921-3927 (2003-08-21)
Mast cells, neutrophils and macrophages are important inflammatory cells that have been implicated in the pathogenesis of acute and chronic inflammatory diseases. To explore a novel anti-inflammatory agent, we have synthesized certain 9-phenoxyacridine and 4-phenoxyfuro[2,3-b]quinoline derivatives and evaluated their anti-inflammatory
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service