134570
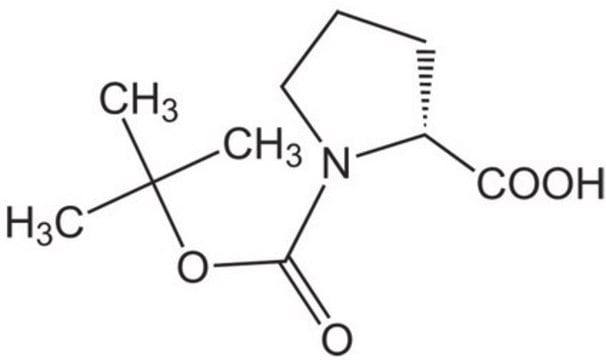
Boc-Pro-OH
99%
Synonym(s):
N-(tert-Butoxycarbonyl)-L-proline, Boc-L-proline
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H17NO4
CAS Number:
Molecular Weight:
215.25
Beilstein:
15828
EC Number:
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
Recommended Products
Assay
99%
form
solid
optical activity
[α]20/D −60°, c = 1 in acetic acid
mp
133-135 °C (lit.)
application(s)
peptide synthesis
SMILES string
CC(C)(C)OC(=O)N1CCC[C@H]1C(O)=O
InChI
1S/C10H17NO4/c1-10(2,3)15-9(14)11-6-4-5-7(11)8(12)13/h7H,4-6H2,1-3H3,(H,12,13)/t7-/m0/s1
InChI key
ZQEBQGAAWMOMAI-ZETCQYMHSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Manos Gkikas et al.
Biomacromolecules, 16(11), 3686-3693 (2015-10-16)
Novel poly(L-lysine)-block-poly(L-proline) (PLL-b-PLP)-based materials with all PLP helical conformers, i.e., PLP II and the rare PLP I are here reported. Electrostatic supramolecular complexation of the adjacent cationic PLL with anionic molecules bearing DNA analogue H-bonding functionalities, such as deoxyguanosine monophosphate
Griet Van Zeebroeck et al.
Nature chemical biology, 5(1), 45-52 (2008-12-09)
Transporter-related nutrient sensors, called transceptors, mediate nutrient activation of signaling pathways through the plasma membrane. The mechanism of action of transporting and nontransporting transceptors is unknown. We have screened 319 amino acid analogs to identify compounds that act on Gap1
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![tert-Butyl 2-oxo-1-oxa-8-azaspiro[4.5]decane-8-carboxylate](/deepweb/assets/sigmaaldrich/product/structures/108/251/f0893bce-f9a2-48e1-bbea-68aaaa08e2e7/640/f0893bce-f9a2-48e1-bbea-68aaaa08e2e7.png)