All Photos(2)
About This Item
Empirical Formula (Hill Notation):
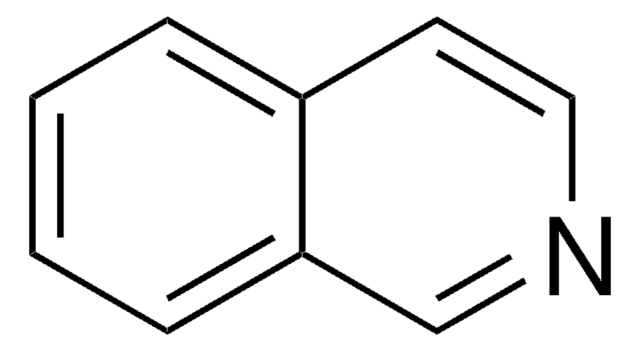
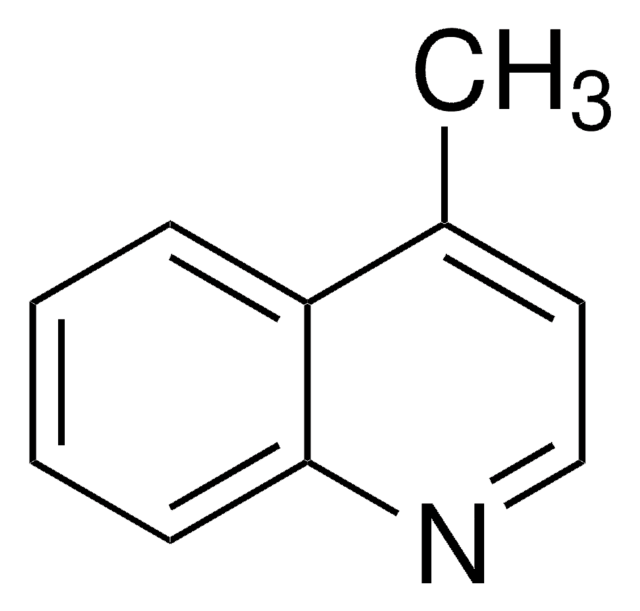
C10H9N
CAS Number:
Molecular Weight:
143.19
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
bp
251 °C (lit.)
mp
63-65 °C (lit.)
SMILES string
Cc1cc2ccccc2cn1
InChI
1S/C10H9N/c1-8-6-9-4-2-3-5-10(9)7-11-8/h2-7H,1H3
InChI key
FVVXWRGARUACNW-UHFFFAOYSA-N
Gene Information
human ... CYP1A2(1544)
Related Categories
General description
The metabolites of 3-methylisoquinoline were separated by adsorption and reversed-phase high-performance liquid chromatography (HPLC).
Application
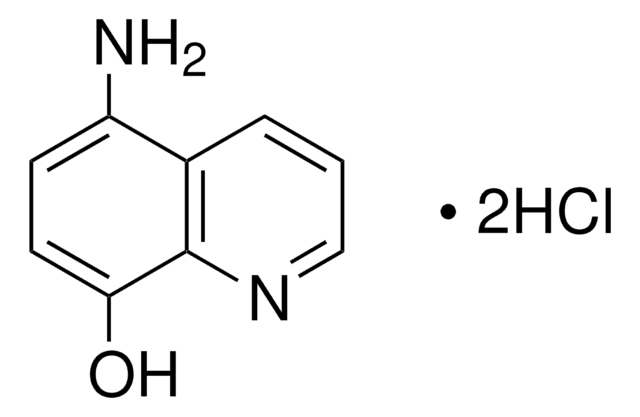
3-Methylisoquinoline was used to prepare 3-aminoisoquinoline.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
C Stubley et al.
Journal of chromatography, 177(2), 313-322 (1979-09-21)
Adsorption and reversed-phase high-performance liquid chromatography (HPLC) have been successfully used to separate metabolites from the parent heterocycles (isoquinoline, 3-methylisoquinoline, phthalazine, quinazoline, quinoxaline and cinnoline). Retention data are reported. The metabolites, hydroxyazanaphthalenes, which arise as a result of aldehyde oxidase
The Preparation of 3-Aminoisoquinoline and Related Compounds1.
Journal of the American Chemical Society, 73(2), 688-689 (1951)
Elisabetta Muntoni et al.
Pharmaceutics, 11(2) (2019-02-06)
Glioblastoma is the most common and invasive primary tumor of the central nervous system and normally has a negative prognosis. Biodistribution in healthy animal models is an important preliminary study aimed at investigating the efficacy of chemotherapy, as it is
Markus Brinkmann et al.
Chemical research in toxicology, 32(4), 698-707 (2019-03-22)
Hydroxylation of polyaromatic compounds through cytochromes P450 (CYPs) is known to result in potentially estrogenic transformation products. Recently, there has been an increasing awareness of the importance of alternative pathways such as aldehyde oxidases (AOX) or N-methyltransferases (NMT) in bioactivation
Kunal Roy et al.
European journal of medicinal chemistry, 44(5), 1941-1951 (2008-12-27)
A series of naphthalene and non-naphthalene derivatives (n=42) having cytochrome P450 2A6 and 2A5 inhibitory activities, reported by Rahnasto et al., were subjected to QSAR and QAAR studies. The analyses were performed using electronic, spatial, shape and thermodynamic descriptors to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service