All Photos(1)
About This Item
Linear Formula:
C5H9C2H5
CAS Number:
Molecular Weight:
98.19
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
>1 (vs air)
vapor pressure
72.8 mmHg ( 37.7 °C)
Assay
98%
autoignition temp.
500 °F
expl. lim.
6.7 %
refractive index
n20/D 1.419 (lit.)
bp
103 °C (lit.)
mp
−138 °C (lit.)
density
0.763 g/mL at 25 °C (lit.)
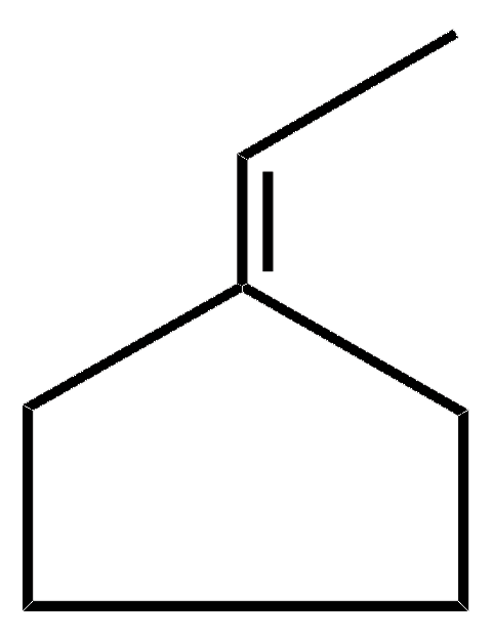
SMILES string
CCC1CCCC1
InChI
1S/C7H14/c1-2-7-5-3-4-6-7/h7H,2-6H2,1H3
InChI key
IFTRQJLVEBNKJK-UHFFFAOYSA-N
Related Categories
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
60.8 °F - closed cup
Flash Point(C)
16 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Gary B McVicker et al.
The journal of physical chemistry. B, 109(6), 2222-2226 (2006-07-21)
In this paper we describe the utility of an acid-catalyzed isomerization reaction, specifically, ring-contraction of methylcyclohexane to an isomeric mixture of alkylcyclopentanes as a tool for characterizing the acidic properties of a wide range of platinum-loaded solid acids. Methylcyclohexane isomerization
Luis A Rios-Hernandez et al.
Applied and environmental microbiology, 69(1), 434-443 (2003-01-07)
We used ethylcyclopentane (ECP) as a model alicyclic hydrocarbon and investigated its metabolism by a sulfate-reducing bacterial enrichment obtained from a gas condensate-contaminated aquifer. The enrichment coupled the consumption of ECP with the stoichiometrically expected amount of sulfate reduced. During
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service