SML3562
TPC2-A1-N
≥98% (HPLC)
Synonym(s):
2-Cyano-3-(3,5-dichlorophenyl)-3-hydroxy-N-(4-(trifluoromethyl)phenyl)acrylamide, TPC2 agonist N19
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C17H9Cl2F3N2O2
CAS Number:
Molecular Weight:
401.17
MDL number:
UNSPSC Code:
12352200
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
powder
color
white to beige
solubility
DMSO: 2 mg/mL, clear
storage temp.
2-8°C
SMILES string
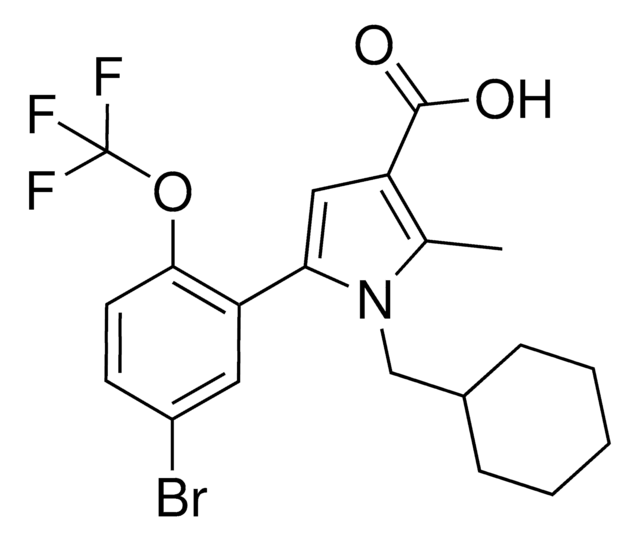
O=C(/C(C#N)=C(O)/C1=CC(Cl)=CC(Cl)=C1)NC2=CC=C(C=C2)C(F)(F)F
Biochem/physiol Actions
TPC2-A1-N is a selective two pore segment channel 2 (TCP2) agonist that mimics the action of NAADP where it selectively induces fast Ca2+ signals (EC50 = 7.8 µM) in a TCP2-dependent manner, without potency toward TRPML1/2/3. In contrast, TPC2-A1-N is a much weaker Na+ current inducer when compared to PI(3,5)P2 and TPC2-A1-P. TPC2-A1-N, but not the Na+ signals agonist TPC2-A1-P causes TPC2-dependent alkalinization of lysosomal lumen Ca2+ stores (10 µM, TPC2-expressing HeLa cells), while TPC2-A1-P, but not TPC2-A1-N, activates TPC2-dependent lysosomal exocytosis (30 µM, murine macrophages).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Estradiol analogs attenuate autophagy, cell migration and invasion by direct and selective inhibition of TRPML1, independent of estrogen receptors
Scientific Reports, 11 (2021)
The lysosomotrope GPN mobilises Ca2+ from acidic organelles
Journal of Cell Science, 134 (2021)
The ethoxycarbonyl group as both activating and protective group in N-acyl-Pictet-Spengler reactions using methoxystyrenes. A short approach to racemic 1-benzyltetrahydroisoquinoline alkaloids
Beilstein Journal of Organic Chemistry, 17 (2021)
Gene editing and synthetically accessible inhibitors reveal role for TPC2 in HCC cell proliferation and tumor growth
Cell Chemical Biology, 28 (2021)
Ramona Schütz et al.
Archiv der Pharmazie, 353(7), e2000106-e2000106 (2020-05-26)
The first racemic total synthesis of the isoquinoline-benzylisoquinoline alkaloid muraricine is reported herein. Pharmacological characterization identified muraricine as a moderate inhibitor of P-glycoprotein, a crucial factor of multidrug resistance in cancer. When combined with vincristine, muraricine partly reversed the chemoresistance of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service