F2929
6-Fluoromevalonate
≥90% (GC), viscous liquid
Synonym(s):
Tetrahydro-4-fluoromethyl-4-hydroxy-2H-pyran-2-one, ZR 3516
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H9FO3
CAS Number:
Molecular Weight:
148.13
MDL number:
UNSPSC Code:
51111800
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥90% (GC)
form
viscous liquid
color
yellow tint
solubility
DMSO: ≥3 mg/mL
storage temp.
2-8°C
SMILES string
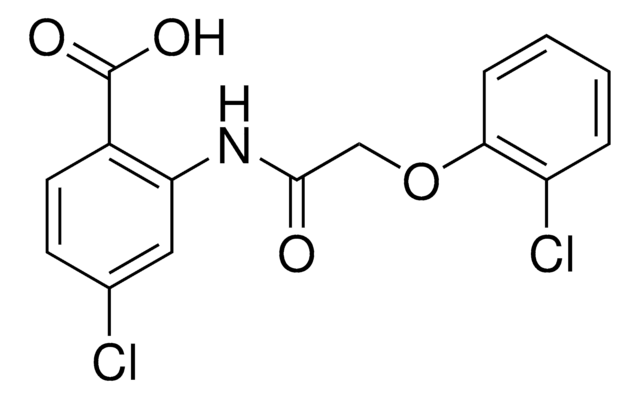
OC1(CF)CCOC(=O)C1
InChI
1S/C6H9FO3/c7-4-6(9)1-2-10-5(8)3-6/h9H,1-4H2
InChI key
DPPMVKMESJJAJZ-UHFFFAOYSA-N
Application
6-Fluoromevalonate has been used as a mevalonate pyrophosphate decarboxylase inhibitor:
- to study the effect of mevalonate pathway inhibition on patient-derived brain tumor-initiating cells (BTICs) growth and self-renewal
- to study its effect on induction of trained immunity by β-glucan in monocytes
- to determine the effect of the mevalonate pathway (MVP) on ADP-ribosylation factor 6 (ARF6) activation
Biochem/physiol Actions
Mevalonate-pyrophosphate decarboxylase inhibitor
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
J A Cuthbert et al.
The Journal of biological chemistry, 265(30), 18568-18575 (1990-10-25)
The sterol synthesis inhibitor 6-fluoromevalonate (Fmev) was used to explore the role of mevalonate products in lymphocyte proliferation. Fmev blocks the synthesis of isopentenyl pyrophosphate and all more distal products in the sterol pathway. When cells were cultured in lipoprotein-deficient
J E Carrel et al.
Experientia, 42(7), 853-854 (1986-07-15)
Biosynthesis of cantharidin in a blister beetle, Lytta polita, is effectively inhibited by 6-fluoromevalonate. Inhibition is attributed specifically to the fluorine substituent. Biochemical inhibition has not been demonstrated previously for an arthropod's defensive substance.
M Sawamura et al.
Clinical and experimental pharmacology & physiology, 20(7-8), 509-514 (1993-07-01)
1. Recent investigations revealed that isoprenoid compounds serve as key substances for cellular proliferation through post-translational modification. Previously we reported that tissues of spontaneously hypertensive rats (SHR) had a lower activity of isoprenoid biosynthesis when compared with the normotensive control
J A Cuthbert et al.
The Journal of biological chemistry, 266(27), 17966-17971 (1991-09-25)
The role of mevalonate and its products in the regulation of cellular proliferation was examined using 6-fluoromevalonate (Fmev), a compound that blocks the conversion of mevalonate pyrophosphate to isopentenyl pyrophosphate. Fmev suppressed DNA synthesis by a variety of transformed and
A Corsini et al.
Comptes rendus des seances de la Societe de biologie et de ses filiales, 191(2), 169-194 (1997-01-01)
The role of mevalonic acid (MVA) and its products (isoprenoids) in cell proliferation prompted us to investigate the effect of drugs affecting diverse enzymatic steps of the MVA pathway on rat aorta smooth muscle cell (SMC) proliferation. Competitive inhibitors of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service