Y0000001
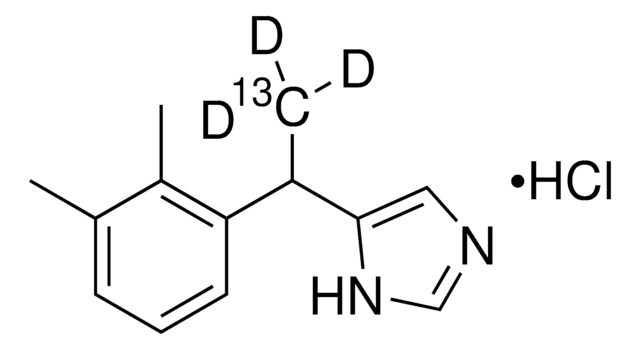
Detomidine hydrochloride
European Pharmacopoeia (EP) Reference Standard
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H14N2 · HCl
CAS Number:
Molecular Weight:
222.71
UNSPSC Code:
41116107
NACRES:
NA.24
Recommended Products
grade
pharmaceutical primary standard
API family
dexmedetomidine, medetomidine
manufacturer/tradename
EDQM
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-8°C
InChI
1S/C12H14N2.ClH/c1-9-4-3-5-11(10(9)2)6-12-7-13-8-14-12;/h3-5,7-8H,6H2,1-2H3,(H,13,14);1H
InChI key
OIWRDXKNDCJZSM-UHFFFAOYSA-N
Related Categories
General description
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Detomidine hydrochloride EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
Packaging
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Other Notes
Sales restrictions may apply.
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
E L M Pimenta et al.
Equine veterinary journal, 43(3), 332-340 (2011-04-16)
Bradycardia may be implicated as a cause of cardiovascular instability during anaesthesia. Hyoscine would induce positive chronotropism of shorter duration than atropine, without adversely impairing intestinal motility in detomidine sedated horses. Ten minutes after detomidine (0.02 mg/kg bwt, i.v.), physiological
Melanie L Church et al.
Veterinary ophthalmology, 15 Suppl 2, 77-83 (2012-04-14)
The main objective was to record electroretinogram (ERG) parameters of normal thoroughbred mares using the HMsERG, a mini-Ganzfeld electroretinographic unit, and a contact lens electrode. The second objective was to determine whether IV detomidine hydrochloride at 0.015 mg/kg is consistently
Valérie Deniau et al.
Veterinary anaesthesia and analgesia, 40(4), 375-381 (2013-02-14)
To compare the changes in splenic length and thickness and in packed cell volume (PCV) following detomidine or xylazine administration and subsequent epinephrine infusion. Spleen relaxation occurs following xylazine or detomidine administration and interferes with subsequent splenic contractile response to
Stijn Schauvliege et al.
Veterinary anaesthesia and analgesia, 38(6), 544-554 (2011-10-13)
To examine the influence of a detomidine constant rate infusion (CRI) on cardiovascular function, isoflurane requirements and recovery quality in horses undergoing elective surgery. Prospective, randomized, blinded, clinical trial. Twenty adult healthy horses. After sedation (detomidine, 10 μg kg(-1) intravenously [IV]) and
Dana L Holve
Journal of the American Veterinary Medical Association, 240(3), 308-311 (2012-01-20)
To determine the effect of sedation with detomidine on intraocular pressure (IOP) in standing horses and whether topical ocular application of anesthetic alters this effect. Clinical trial. 15 clinically normal horses. Horses were assigned to group 1 (n = 7)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service