L3900
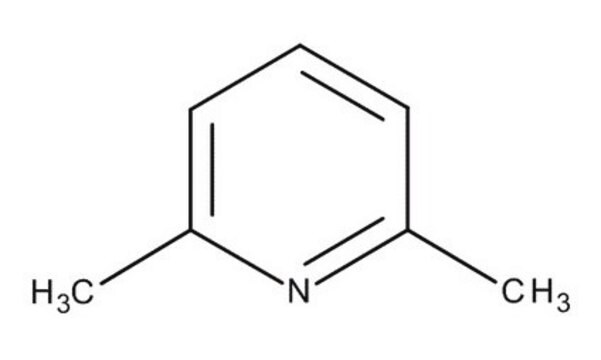
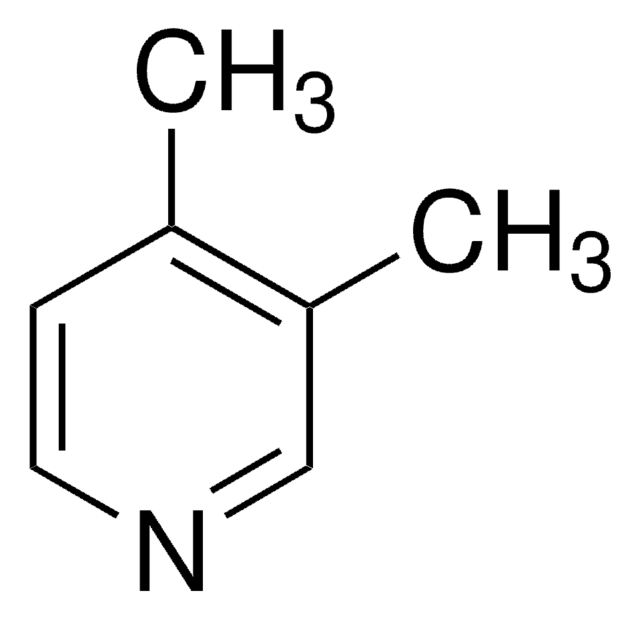
2,6-Lutidine
ReagentPlus®, 98%
Synonym(s):
2,6-Dimethylpyridine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C7H9N
CAS Number:
Molecular Weight:
107.15
Beilstein:
105690
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
98%
refractive index
n20/D 1.497 (lit.)
bp
143-145 °C (lit.)
mp
−6 °C (lit.)
density
0.92 g/mL at 25 °C (lit.)
SMILES string
Cc1cccc(C)n1
InChI
1S/C7H9N/c1-6-4-3-5-7(2)8-6/h3-5H,1-2H3
InChI key
OISVCGZHLKNMSJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
2,6-Lutidine can be used as a base for the oxidation of α-CF3 alcohols to the corresponding carbonyl compounds in the presence of 4-acetamido-2,2,6,6-tetramethylpiperidine-1-oxoammonium tetrafluoroborate (Bobbitt′s salt) as oxidation reagent.
It can be used:
It can be used:
- As a promoter for catalytic asymmetric fluorination of α-cyanophosphonates in the presence of chiral Pd(II)-bisphosphine complexes.
- In combination with tert-butyldimethylsilyl triflate for the protection of tertiary alcohols and unreactive secondary alcohols.
- In combination with triethylsilyl trifluoromethanesulfonate for the conversion of acetals to the corresponding aldehydes in dichloromethane followed by workup in water.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
91.4 °F
Flash Point(C)
33 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Unexpected Highly Chemoselective Deprotection of the Acetals from Aldehydes and Not Ketones: TESOTf? 2, 6-Lutidine Combination.
Fujioka H, et al.
Journal of the American Chemical Society, 126(38), 11800-11801 (2004)
Pd (II)-Catalyzed Asymmetric Fluorination of ?-Aryl-?-cyanophosphonates with the Aid of 2, 6-Lutidine.
Moriya K I, et al.
Synlett, 2007(07), 1139-1142 (2007)
Studies with trialkylsilyltriflates: new syntheses and applications.
Corey E J, et al.
Tetrahedron Letters, 22(36), 3455-3458 (1981)
Synthesis of 4-acetamido-2, 2, 6, 6-tetramethylpiperidine-1-oxoammonium tetrafluoroborate and 4-acetamido-(2, 2, 6, 6-tetramethyl-piperidin-1-yl) oxyl and their use in oxidative reactions.
Mercadante M A, et al.
Nature Protocols, 8(4), 666-666 (2013)
Bojana Ginovska et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 21(44), 15713-15719 (2015-10-24)
We report that 2,6-lutidine⋅trichloroborane (Lut⋅BCl3 ) reacts with H2 in toluene, bromobenzene, dichloromethane, and Lut solvents producing the neutral hydride, Lut⋅BHCl2 . The mechanism was modeled with density functional theory, and energies of stationary states were calculated at the G3(MP2)B3
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service