14335
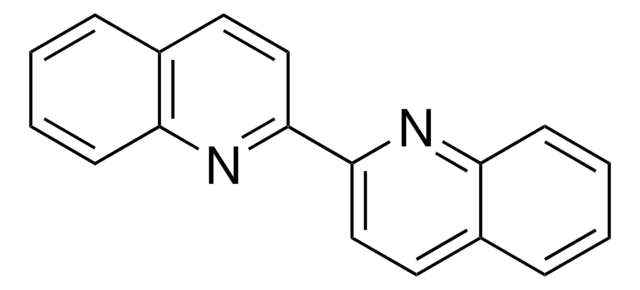
2,2′-Biquinoline-4,4′-dicarboxylic acid
≥90% (TLC)
Synonym(s):
2,2′-Bicinchoninic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C20H12N2O4
CAS Number:
Molecular Weight:
344.32
Beilstein:
321561
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Quality Level
Assay
≥90% (TLC)
form
powder
storage temp.
2-8°C
SMILES string
OC(=O)c1cc(nc2ccccc12)-c3cc(C(O)=O)c4ccccc4n3
InChI
1S/C20H12N2O4/c23-19(24)13-9-17(21-15-7-3-1-5-11(13)15)18-10-14(20(25)26)12-6-2-4-8-16(12)22-18/h1-10H,(H,23,24)(H,25,26)
InChI key
AFYNADDZULBEJA-UHFFFAOYSA-N
Application
2,2′-Biquinoline-4,4′-dicarboxylic acid has been used in a study that synthesized and structurally characterized six metal-organic coordination polymers.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C Faye et al.
Analytical biochemistry, 420(2), 147-154 (2011-10-11)
The efficient immobilization of antibodies on monolithic support is one of the most critical steps when preparing immunoaffinity supports. In this work, the ADECA (amino density estimation by colorimetric assay) method was adapted to tridimensional supports (in a dynamic mode)
Baoyan Bai et al.
Proteomics, 12(19-20), 3044-3048 (2012-08-15)
Efficient extraction and accurate quantification of nucleolar macromolecules are critical for in vitro analysis, especially for studying RNA, DNA, and protein dynamics under identical conditions. There is presently no single method that efficiently and simultaneously isolates these three macromolecular constituents
Randall I Krohn
Current protocols in cell biology, Appendix 3, 3H-3H (2011-09-08)
Protein quantification is an important step for handling protein samples for isolation and characterization; it is a prerequisite step before submitting proteins for chromatographic, electrophoretic, or immunochemical analysis and separation. Colorimetric methods are fast, simple, and not laborious. This unit
Jennifer R Weiser et al.
Analytical biochemistry, 430(2), 116-122 (2012-08-23)
A new class of compounds amenable to quantification by the bicinchoninic acid (BCA) assay was identified, allowing an expansion of compounds quantifiable within the assay's capacity. In this article, we demonstrate that compounds containing the α-hydroxy ketone structure are easily
C Petrella et al.
Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society, 22(11), 1248-1256 (2010-07-28)
Cannabinoids (CBs) evoke their effects by activating the cannabinoid receptor subtypes CB1-r and CB2-r and exert anti-inflammatory effects altering chemokine and cytokine expression. Various cytokines and chemokines are produced and released by rodent pancreatic acini in acute pancreatitis. Although CB1-r
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service