03951
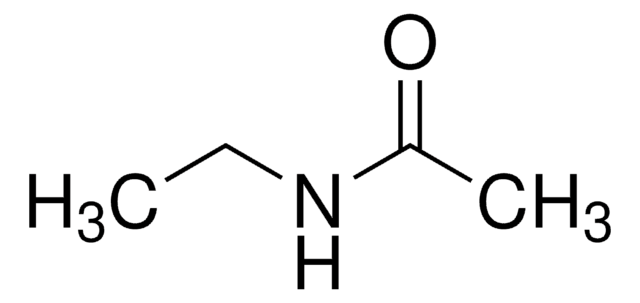
N-Ethylformamide
≥99.0% (GC)
Synonym(s):
N-Formylethylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HCONHC2H5
CAS Number:
Molecular Weight:
73.09
Beilstein:
1737860
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
100
500
Assay
≥99.0% (GC)
form
liquid
refractive index
n20/D 1.432
bp
202-204 °C
density
0.950 g/mL at 20 °C (lit.)
SMILES string
CCNC=O
InChI
1S/C3H7NO/c1-2-4-3-5/h3H,2H2,1H3,(H,4,5)
InChI key
KERBAAIBDHEFDD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
H Cross et al.
Chemical research in toxicology, 3(4), 357-362 (1990-07-01)
Hepatotoxic formamides such as N-methylformamide (NMF) and N,N-dimethylformamide (DMF) are metabolized in vivo to N-acetyl-S-(N-methylcarbamoyl)cysteine via oxidation at the formyl carbon, which yields a reactive intermediate. The hypothesis was tested that this biotransformation route can be studied in vitro with
M R Del Carratore et al.
Environmental and molecular mutagenesis, 36(2), 97-104 (2000-10-03)
A cDNA coding for rat cytochrome P450 2E1 was cloned into the multicopy vector pYeDP60 and expressed in haploid RSY6 and diploid RS112 yeast strains of Saccharomyces cerevisiae under control of the GAL10-CYC1 promoter. Spectral and catalytic properties of the
P Kestell et al.
The Journal of pharmacology and experimental therapeutics, 240(1), 265-270 (1987-01-01)
The hepatotoxicity and metabolism of the following close analogs of the hepatotoxic antitumor agent N-methylformamide (NMF) were investigated in CBA/CA mice: N-ethylformamide (NEF), dimethylformamide (DMF), formamide and N-methylacetamide (NMA). Apart from NMF only NEF was potently hepatotoxic as measured by
S P Langdon et al.
Toxicology, 43(3), 239-249 (1987-03-01)
The induction of terminal differentiation in tumour cells represents a possible therapeutic strategy for treating cancer. The alkylformamides are 1 group of experimental compounds which have been shown to induce terminal differentiation in human HL-60 leukemia and murine Friend erythroleukemia
Gil-Soo Han et al.
The Journal of biological chemistry, 283(29), 20443-20453 (2008-05-07)
The Saccharomyces cerevisiae DGK1 gene encodes a diacylglycerol kinase enzyme that catalyzes the formation of phosphatidate from diacylglycerol. Unlike the diacylglycerol kinases from bacteria, plants, and animals, the yeast enzyme utilizes CTP, instead of ATP, as the phosphate donor in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service