MAB5450
Anti-Tau Antibody, phosphoThreonine 231, clone PHF-6
ascites fluid, clone PHF-6, Chemicon®
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352203
eCl@ss:
32160702
NACRES:
NA.41
Recommended Products
biological source
mouse
antibody form
ascites fluid
clone
PHF-6, monoclonal
species reactivity
human
manufacturer/tradename
Chemicon®
technique(s)
ELISA: suitable
western blot: suitable
isotype
IgG1
NCBI accession no.
UniProt accession no.
shipped in
dry ice
target post-translational modification
phosphorylation (pThr231)
Specificity
Reacts with human Tau phosphorylated at threonine 231 and fetal tau. The antibody also reacts with dephosphorylated neurofibrillary tangles. MAB5450 is reactive with the Thr231 phosphorylated and diphosphorylated peptides. No reactivity with normal adult tau or with unphosphorylated or serine 235 phosphorylated protein.
Immunogen
Epitope: phosphoThreonine 231
Paired helical filaments tau preparation from human brain.
Application
Anti-Tau Antibody, phosphoThreonine 231, clone PHF-6 is an antibody against Tau for use in ELISA & WB.
Research Category
Neuroscience
Neuroscience
Research Sub Category
Neurodegenerative Diseases
Neurodegenerative Diseases
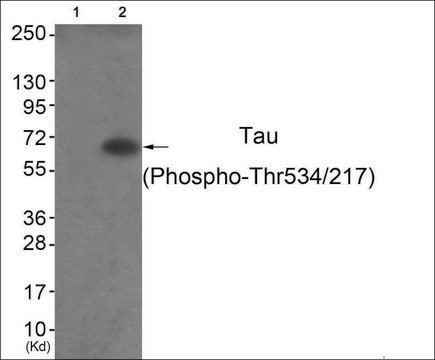
Western blot: 1:1000, non-phosphate buffers recommended. Specific for phospho-tau however highly phosphorylated blocking materials like non-fat milk can sometimes cause difficulties, thus we generally recommend blocking western blots with TBS-1-2% BSA solutions (filtered through a 0.45μm membrane) for better results.
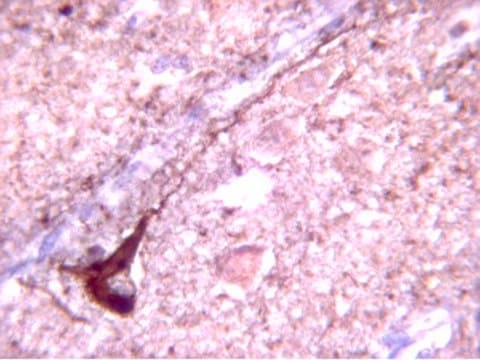
Immunohistochemistry: fresh frozen tissues with Tris-NaCl-Triton treatment
{http://www.jhc.org/cgi/content/full/48/12/1627} & http://ajp.amjpathol.org/cgi/content/full/160/6/2045
Optimal working dilutions must be determined by end user.
Immunohistochemistry: fresh frozen tissues with Tris-NaCl-Triton treatment
{http://www.jhc.org/cgi/content/full/48/12/1627} & http://ajp.amjpathol.org/cgi/content/full/160/6/2045
Optimal working dilutions must be determined by end user.
Physical form
Liquid.
Storage and Stability
Maintain at -20°C in undiluted aliquots for up to 12 months after date of receipt. Avoid repeated freeze/thaw cycles.
Legal Information
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
G T Bramblett et al.
Neuron, 10(6), 1089-1099 (1993-06-01)
Abnormally phosphorylated tau proteins (A68) are the building blocks of Alzheimer's disease (AD) paired helical filaments. The biological consequences of the conversion of normal adult tau to A68 remain unknown. Here we demonstrate that native A68 does not bind to
Chien-Ning Huang et al.
BMC complementary medicine and therapies, 20(1), 370-370 (2020-12-04)
Insulin resistance could be associated with the development of Alzheimer disease (AD). The neuropathological hallmarks of AD are beta amyloid (Aβ) produced from sequential cleavage initiated by β-secretase and degraded by insulin degradation enzyme (IDE), as well as hyperphosphorylation of
Quantitative phosphoproteomics of Alzheimer's disease reveals cross-talk between kinases and small heat shock proteins.
Dammer, EB; Lee, AK; Duong, DM; Gearing, M; Lah, JJ; Levey, AI; Seyfried, NT
Proteomics null
Pablo Martinez et al.
Nature neuroscience, 25(12), 1597-1607 (2022-11-08)
Tau aggregation is a defining histopathological feature of Alzheimer's disease and other tauopathies. However, the cellular mechanisms involved in tau propagation remain unclear. Here, we performed an unbiased quantitative proteomic study to identify proteins that specifically interact with this tau
Chadwick M Hales et al.
Brain pathology (Zurich, Switzerland), 24(4), 344-351 (2014-02-28)
We recently discovered that protein components of the ribonucleic acid (RNA) spliceosome form cytoplasmic aggregates in Alzheimer's disease (AD) brain, resulting in widespread changes in RNA splicing. However, the involvement of small nuclear RNAs (snRNAs), also key components of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service