AB3561
Anti-phospho HSP27 (pS82) Antibody
Chemicon®, from rabbit
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352203
eCl@ss:
32160702
NACRES:
NA.41
Recommended Products
biological source
rabbit
Quality Level
antibody form
purified immunoglobulin
antibody product type
primary antibodies
clone
polyclonal
species reactivity
human
manufacturer/tradename
Chemicon®
technique(s)
western blot: suitable
NCBI accession no.
UniProt accession no.
shipped in
dry ice
target post-translational modification
phosphorylation (pSer82)
Gene Information
human ... HSPB1(3315)
General description
Heat Shock Protein 27 (HSP27) is a 27 kDa member of a family of proteins whose expression and function are stimulated by heat shock and other stress stimuli. A major function of these proteins is to serve as chaperones that bind to and stabilize the active conformation of other proteins. HSP27, along with other members of the small HSP group, possesses a C-terminal α-crystalline homology domain. HSP27 is localized to the cytoplasm of unstressed cells but can redistribute to the nucleus in response to stress, where it may function to stabilize DNA and/or the nuclear membrane. Cytoplasmic HSP27 exists in multiple complexes. One complex consists of HSP27, Akt (PKB), MAPKAP-kinase 2, and p38 MAPK. The presence of HSP27 in this complex is required for Akt activation by stress stimuli. Another complex consists of HSP27 and the IKK complex. HSP27 is also an actin capping protein that binds to the barbed (growing) ends of actin filaments, thereby inhibiting filament extension. Phosphorylation of HSP27 on serine 82 by MAPKAP-kinase 2 leads to HSP27 dissociation from the Akt/MAPKAP-kinase 2/p38 complex and from actin filaments, and stimulates HSP27 binding to the IKK complex.
Specificity
Human HSP27. This antibody does not detect endogenous mouse HSP25 [pS86] protein in extracts of NIH3T3 cells treated with anisomycin; however, interaction with the human HSP27 protein is inhibited by the phosphopeptide used to generate the mouse HSP25 [pS86] antibody.
Immunogen
The antiserum was produced against a chemically synthesized phosphopeptide derived from a region of human HSP27 that contains serine 82.
Application
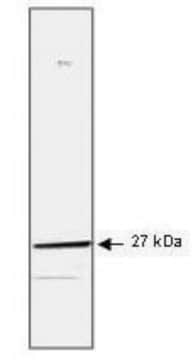
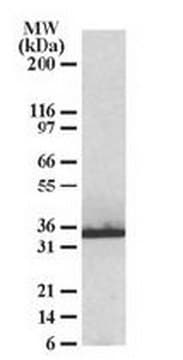
Anti-phospho HSP27 (pS82) Antibody detects level of phospho HSP27 (pS82) & has been published & validated for use in WB.
Research Category
Protein Trafficking
Protein Trafficking
Research Sub Category
Chaperones
Chaperones
Linkage
Replaces: 04-448
Physical form
Dulbecco′s phosphate buffered saline (without Mg2+ and Ca2+), pH 7.3 (+/- 0.1), 50% glycerol with 1.0 mg/mL BSA (IgG, protease free) as a carrier. 0.05% sodium azide.
Format: Purified
Storage and Stability
Store at -20°C. We recommend a brief centrifugation before opening to settle vial contents. Then, apportion into working aliquots and store at -20°C. For shipment or short-term storage (up to one week), 2-8°C is sufficient.
Analysis Note
Control
HeLa cells treated with TNF-α.
HeLa cells treated with TNF-α.
Legal Information
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Not finding the right product?
Try our Product Selector Tool.
recommended
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service