All Photos(1)
About This Item
Empirical Formula (Hill Notation):
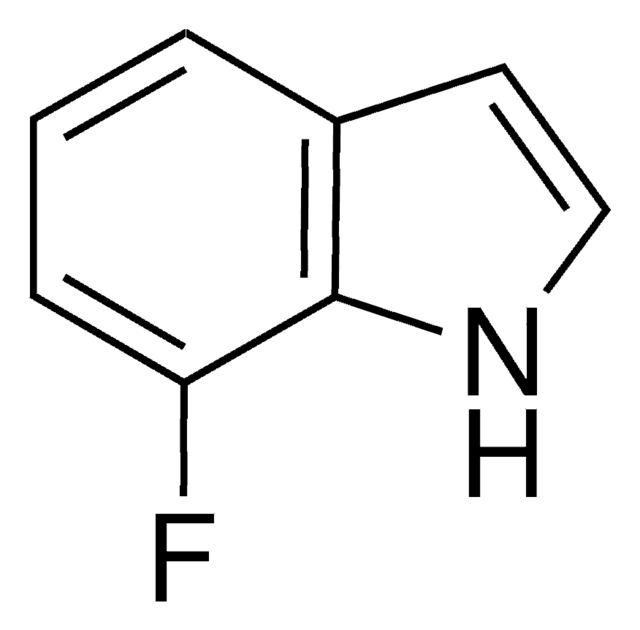
C8H6FN
CAS Number:
Molecular Weight:
135.14
Beilstein:
112350
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
45-48 °C (lit.)
SMILES string
Fc1ccc2[nH]ccc2c1
InChI
1S/C8H6FN/c9-7-1-2-8-6(5-7)3-4-10-8/h1-5,10H
InChI key
ODFFPRGJZRXNHZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Reactant for preparation of 5-HT6 receptor ligands
- Reactant for preparation of tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Reactant for preparation of antitumor agents
- Reactant for preparation of antibacterial agents
- Reactant for preparation of immunosuppressive agents
- Reactant for preparation of Sodium-Dependent Glucose Co-transporter 2 (SGLT2) Inhibitors for the Management of Hyperglycemia in Diabetes
- Reactant for preparation of Myeloperoxidase Inhibitors
- Reactant for preparation of Potent Selective Serotonin Reuptake Inhibitors
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Nicholas A Magnus et al.
Organic letters, 12(16), 3700-3703 (2010-08-14)
A practical synthesis of the glycogen synthase kinase-3 (GSK3) inhibitor bisarylmaleimide 1 has been accomplished employing Pictet-Spengler methodology to access the indole 7-position in preparing the benzodiazepine tricyclic fragment. A seven-step linear sequence that starts with commercially available 5-fluoroindole 7
A L Palombella et al.
Plant physiology, 117(2), 455-464 (1998-06-25)
We report the isolation of a Chlamydomonas reinhardtii cDNA that encodes the beta-subunit of tryptophan synthase (TSB). This cDNA was cloned by functional complementation of a trp-operon-deleted strain of Escherichia coli. Hybridization analysis indicated that the gene exists in a
Steffen P Graether et al.
Journal of magnetic resonance (San Diego, Calif. : 1997), 178(1), 65-71 (2005-10-04)
We show that it is feasible to use a minicoil for solid-state 19F 1H NMR experiments that has short pulse widths, good RF homogeneity, and excellent signal-to-noise for small samples while using low power amplifiers typical to liquid-state NMR. The
Peter B Crowley et al.
Chemical communications (Cambridge, England), 48(86), 10681-10683 (2012-09-25)
Fluorine-containing amino acids are valuable probes for the biophysical characterization of proteins. Current methods for (19)F-labeled protein production involve time-consuming genetic manipulation, compromised expression systems and expensive reagents. We show that Escherichia coli BL21, the workhorse of protein production, can
A J Barczak et al.
Genetics, 140(1), 303-313 (1995-05-01)
A study of the biochemical genetics of the Arabidopsis thaliana tryptophan synthase beta subunit was initiated by characterization of mutants resistant to the inhibitor 5-fluoroindole. Thirteen recessive mutations were recovered that are allelic to trp2-1, a mutation in the more
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service