All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H18
CAS Number:
Molecular Weight:
138.25
Beilstein:
4364365
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98.0%
form
liquid
SMILES string
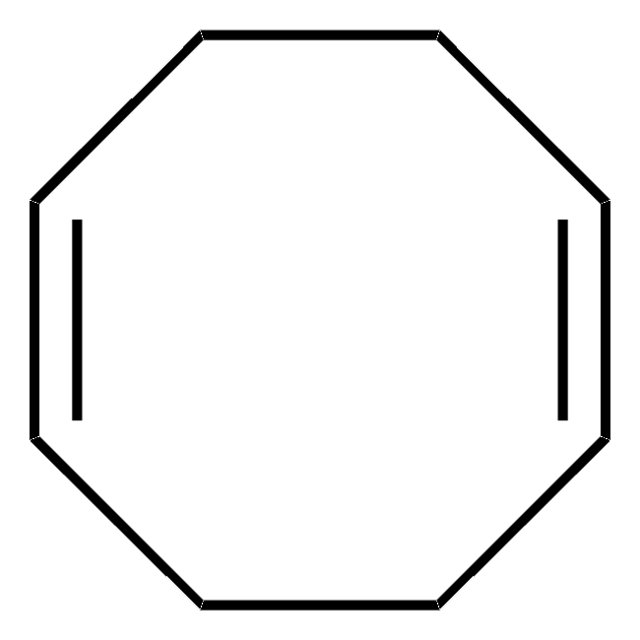
C=CC1CCCCCCC1
InChI
1S/C10H18/c1-2-10-8-6-4-3-5-7-9-10/h2,10H,1,3-9H2
InChI key
UPHVHMSLLBDZEV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
69.8 °F - closed cup
Flash Point(C)
21 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
P G Gervasi et al.
Toxicology letters, 20(3), 243-249 (1984-03-01)
Vinylcyclooctane (VCO), which binds to the active site of cytochrome P-450 (P-450) giving a type I difference spectrum, has been found to form the corresponding epoxide as the main metabolite on treatment with liver microsomal monooxygenase obtained from phenobarbital-treated or
P G Gervasi et al.
Toxicology letters, 16(3-4), 217-223 (1983-05-01)
Vinylcyclooctane, when administered to mice at 500 mg/kg, produced reduction of microsomal cytochrome P-450, heme, aminopyrine-N-demethylase and ethoxycoumarin-O-deethylase activities with respect to control values; furthermore the hepatic reduced glutathione level was depleted suggesting that glutathione is involved in the vinylcyclooctane
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service