All Photos(1)
About This Item
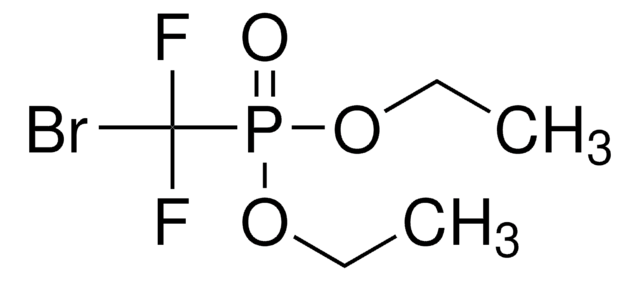
Linear Formula:
(O2N)2C6H3NCO
CAS Number:
Molecular Weight:
209.12
Beilstein:
3097358
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
95%
mp
82-87 °C (lit.)
storage temp.
2-8°C
SMILES string
[O-][N+](=O)c1cc(cc(c1)[N+]([O-])=O)N=C=O
InChI
1S/C7H3N3O5/c11-4-8-5-1-6(9(12)13)3-7(2-5)10(14)15/h1-3H
InChI key
JZPRXQSCMBDGKP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
High-performance liquid chromatographic separation of chiral alcohols on chiral stationary phases.
Oi N and Kitahara H.
Journal of Chromatography A, 265, 117-120 (1983)
Anthracene derivatives bearing two urea groups as fluorescent receptors for anions.
Kim SK, et al.
Tetrahedron, 61(19), 4545-4550 (2005)
Sadegh Yaghoubnejad et al.
Journal of separation science, 41(9), 1903-1912 (2018-01-16)
We report the synthesis and enantioseparation characteristics of two novel covalently immobilized deoxycholic acid derivatives as chiral stationary phases for high-performance liquid chromatography. In the structure of the first stationary phase, the 3-position of deoxycholic acid is substituted with a
J N Zeng et al.
Journal of chromatography. B, Biomedical applications, 654(2), 231-248 (1994-04-01)
A new chiral high-performance liquid chromatographic (HPLC) method utilizing ultraviolet (UV) detection has been developed for determining plasma and urinary concentrations of d-fenfluramine and its major metabolite d-norfenfluramine, while being able to determine the possible presence of l-fenfluramine after oral
A J Bourque et al.
Journal of pharmaceutical and biomedical analysis, 11(6), 495-503 (1993-06-01)
Solid-phase derivatization reagents containing a 3,5-dinitrophenyl moiety for the derivatization of amines are described. The reagents are useful for Pirkle-type recognition of stereochemical composition of amines and related nucleophiles. The ability to stabilize 3,5-dinitrophenylisocyanate by covalent immobilization on a polymeric
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service